Abstract
Introduction
Organic carbon (OC) fractions play important roles in soil and many ecosystem processes. This study focuses on changing of OC in density and soluble fractions in a soil amended by nanozeolite and plant residues that incubated in lab condition for 90 days.
Results
The results showed that amounts of OC in light fraction (LF) and heavy fraction (HF) increased with the increasing percentage of nanozeolite and plant residues in the soil. The highest amounts of LF (7.54 g LF. kg-1 Soil) and HF (11.10 g kg-1 Soil) were found when 30 % nanozeolite and 5 % wheat and alfalfa straws were added to the soil. Accordingly, wheat and alfalfa straws were effective on increasing the LF and HF, respectively. However, they decreased with declining the OC from the 1st day of experiment until the 90th day of experiment. Soluble OC in hot (2.22 g kg-1 Soil) and cool (1.54 g kg-1 Soil) water fractions increased by addition of 30 % nanozeolite and 5 % plant residues particularly alfalfa straw in comparison with control. Although these soluble fractions increased after initial 30 days of incubation, they decreased in the continuation of the experiment.
Conclusion
In fact, OC contents in density and soluble fractions increased by addition of 30 % nanozeolite and 5 % plant residues into the soil; however, they decreased in initial 30 days of incubation with declining the OC. The findings of this research revealed the application of nanozeolite and plant residues improved carbon pools in density and soluble fractions and carbon sequestration increased by increasing OC contents in soil.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It has been recognized in last decades that the amounts of carbon stored in soils have important effects on a global scale. Therefore, land management practices affecting soil organic carbon (SOC) content may have a global impact, if they are applied over large areas (Bronick and Lal 2005). Therefore, the small fluctuations of SOC may potentially alter the atmospheric carbon dioxide (CO2) concentration and the global climate (Mahmoodabadi and Heydarpour 2014). The carbon storage and rate of CO2 sequestration in soils depend on climate, soil properties and management. Soils as a sink for atmospheric CO2 play a key role in the global carbon budget as well as in the global carbon cycle (Eshel et al. 2007). Soils are known as one of the largest active carbon pools after the hydrosphere and the lithosphere. The role of soils as either a source or a sink for greenhouse gases, in general, and that of CO2, in particular, has been a focus of recent studies (Majumder et al. 2008; Bhattacharyya et al. 2009).
Whereas the largest terrestrial pool of carbon is located in the soils (Bhattacharyya et al. 2009), there are many factors that influence on carbon retention and release in soil and also carbon exchange between soil and atmosphere (Majumder et al. 2008). Storage of organic carbon (OC) in agricultural systems is a balance between carbon additions from non-harvested portions of crops (Wang et al. 2011), organic sources (Thelen et al. 2010) and carbon losses, primarily through organic matter decomposition and release of respired CO2 to the atmosphere (Bird et al. 2002). Last researches have shown that organic substances improve soil aggregation and consequently soil structure and also diminish soil compaction and surface crusting, enlarge carbon sequestration and nutrient availability, and increase infiltration and water-holding capacity (Balashov et al. 2010; Aminiyan et al. 2015).
Density fractionation
Physical fractionation, in contrast to chemical fractionation, allows for isolation of fractions, can isolate as intact as possible the SOC associated predominantly with soil minerals (primary organo-mineral complexes) and also the SOC protected within aggregates (secondary organo-mineral complexes) due to their three-dimensional architecture (Straathof et al. 2014). OC fractions exhibit different rates of biochemical and microbial degradation (Stevenson 1994; Tan et al. 2007) as well as different accessibility and interactions (Sollins et al. 1999). Very little is known of the dynamics of soil organic matter (SOM) after agricultural abandonment. SOC contains fractions with a rapid turnover rate as well as fractions with a slower turnover rate (Aminiyan et al. 2015). The labile fractions of OC, such as microbial biomass carbon (MBC) and dissolved organic carbon (DOC), can respond rapidly to changes in C supply. The dynamics of SOC are usually described by dividing SOM into two or more fractions. Density fractionation, that is a laboratory procedure, physically separates soil into light (LF) and heavy fractions (HF) (Wander and Traina 1996; Sollins et al. 1999). The procedure is useful method for studying labile pools of SOC that are more sensitive to cropping practice than is the total SOC pool in temperate soils (Janzen et al. 1992). Generally, sodium polytungstate (SPT) (1.85 g mL−1) and NaI (1.3 g cm−3) solutions are often used for density fractionation (Golchin et al. 1994; Magid et al. 1996; Six et al. 2002). Although LF is commonly referred to a plant-like and less stable fraction with high OC concentration (Gregorich et al. 2003), HF is more stable and high-density organo-mineral fractions having lower C concentrations (Golchin et al. 1995a, b).
Light fraction of SOM not only is sensitive to changes in management practices (Cambardella and Elliott 1992; Bremer et al. 1994) but also correlates well with the rate of nitrogen (N) mineralization (Hassink 1995; Barrios et al. 1996). The importance of LF (including free and occluded organic C within aggregates) is widely recognized for its role in formation and stability of soil structure, especially in stabilization of soil macroaggregates (>250 mm) (Miller and Jastrow 1990; Kay 1998). Janzen et al. (1992) concluded that LF in surface soil (0–7.5 cm) accounted for 2–17 % of SOC, depending principally on cropping systems. However, there are few studies about these two fractions and their contributions to OC storage as related to changes soil management systems.
Water-soluble organic carbon
Dissolved organic carbon (DOC) is defined as a mixture of organic molecules produced by the decomposition of SOM and plant material and by root exudation (Strobel et al. 2001). Although it has been assumed that dissolved organic matter (DOM) represents a labile part of SOM and that total DOC concentration and especially its easily degradable part resembles soil microbial activity, it has been suggested that a great part of DOM in soil represents a relatively stable by-product of microbial activity (Zhao et al. 2008; Wang et al. 2013). The movement of DOM is significant to the cycling and distribution of nutrients and carbon within and between ecosystems and contributes to soil forming processes (Kalbitz et al. 2003). Plant residue and humus are the most significant sources of soluble organic matter in soil. Gregorich et al. (2000) hypothesized that, although the water-soluble carbon pool was small, it had a high turnover and was in equilibrium with soil humus. Liang et al. (2012) suggested that the difference in water-soluble and biodegradable C in agricultural soils was greater than forest soils due to increases in soluble humic materials in agricultural soils. Therefore, humus is probably the main source of DOC because of the relatively large amount of humus present in soil relative to that contributed by the microbial biomass or recently deposited plant residues. DOC inputs to soil solution originate from biological decomposition, throughfall or litter leaching, root exudates and from deposition of soot and dust (Wang et al. 2013).
Laboratory studies (Smolander and Kitunen 2011; Liang et al. 2012; Kiikkilä et al. 2014) have shown that microorganisms can decompose many amounts of water-soluble organic matter fraction. These studies, which ranged in duration from hours to months, indicated that 10–40 % of the water-soluble OC was decomposable under laboratory conditions. Liang et al. (2012) reported that SOC had significantly positive correlations with labile organic C fractions in the 0–20 cm depth. Smolander and Kitunen (2011) observed positive correlations between DOC concentration extracted from soil and the rate of C and net N mineralization and amount of C and N in microbial biomass, which were used to assess soil microbial activity. High temperature is known to hydrolyze organic structures, lyse cells and dissociate organic materials from inorganic colloids (Kiikkilä et al. 2014). Kim et al. (2014) reported strong correlations between organic C extracted with hot water and mineralizable carbon. This easily degradable DOM seemed to decrease fairly consistently during the degradation of organic matter as observed previously (Don and Kalbitz 2005; Sanderman et al. 2008).
During last years, great strides have been made in a number of research topics including characterizing the spatial and temporal variations in the concentration and flux of DOC reviewed by Kalbitz et al. 2000 and Aitkenhead-Peterson et al. 2003, quantifying its role in soil chemistry and pedogenesis (e.g., Kaiser and Zech 1998; Jansen et al. 2003; Cances et al. 2003), describing the chemical composition of DOC (Guggenberger and Zech 1994; Kaiser and Zech 1998; Strobel et al. 2001), and quantifying the availability of DOC to soil microflora (Zsolnay and Gorlitz 1994; Yano et al. 2000; Kalbitz et al. 2003; Marschner and Kalbitz 2003). There are few studies about assaying of zeolitic materials effects on SOC. Zeolitic materials are extensively used to improve soil physical environment, particularly, in sandy and clay poor soils (Abdi et al. 2006). The assessment of nanozeolite effects on SOC showed that the addition of higher percentage of nanozeolite with alfalfa straw into the soil increased SOC pools and improved soil aggregation stability (Aminiyan et al. 2015). The main objectives of this study were to determine water-soluble and density fractions of OC in soils that treated by different percentage of nanozeolite and some plant residues and incubation them in field capacity for 90 days.
Materials and methods
Study area
This study was conducted on agricultural soil in Azandariyan, Hamedan province, the west of Iran. This area was located between longitudes 47°42′ and 48°45′ E and latitudes 33°28′ and 34°29′ N. The climate of the region is semiarid with a mean annual precipitation of 300 mm and a mean annual temperature of 10 °C. The soil of the area is mostly classified as Typic Haplocalcids (Aminiyan et al. 2015).
Sampling, treatment and analysis of soil
The methods used for soil sampling, treatment and analysis were reported in Aminiyan et al. (2015). The treated and moistened soils were incubated in laboratory condition (20–25 °C) for 90 days. After 1, 5, 10, 20, 30, 45, 60, 75 and 90 days of incubation, a portion of each soil was taken for the study of in density (light and heavy) and soluble (hot water and cool water) OC fractions.
Density fractionation
About 10 g dried sample was transferred to a 20-ml graduated centrifuge tube, and 50 ml of NaI solution (d = 1.3 g cm−3) was added. Suspensions were immediately centrifuged at 3000 rpm for 10 min. The supernatant containing the LF was decanted onto Whatman no. 50 filters (2.7-p.m. retention) and vacuum-filtered. The HF residue was re-suspended twice in fresh NaI solution, and the LFs were combined. LF and HF were then washed four times into pre weighed tins with deionized water, afterward dried at 55 °C for 24 h in the oven, and weighed (Sollins et al. 1999). Then OC content in HF was determined by Walkley and Black (1934) method.
Soluble water organic carbon fractions
The soluble water OC in the whole soil and the three aggregate fractions were extracted using cold water followed by hot water. Soluble organic matter in cold water was extracted from soils by adding 150 ml of distilled/deionized water to a tube containing 15 g of air-dried whole soil or aggregate fraction. The soil water suspension was shaken for 30 min and centrifuged at 4500 rpm for 20 min. The supernatant solutions were decanted and passed through a 0.45-µm cellulose nitrate filter. The weight of extraction tubes with remaining wet soil was recorded in order to calculate the amount of cold water extract remaining. Hot water-soluble organic matter was extracted from these soils by adding water to the wet soil remaining in each tube to return the water volume to 150 ml, then by placing the tubes in a water bath at 80 °C for 16 h. After this period of time, the samples were centrifuged, decanted and filtered as above. Filtered solutions were stored in a refrigerator (4 °C) prior to incubation (Gregorich et al. 2003). Then OC content in HF was determined by Walkley and Black (1934) method.
Statistical data analysis
The experiment was a completely randomized factorial design with three replicates. The factors applied were alfalfa straw (0 and 5 %), wheat straw (0 and 5 %), nanozeolite (0, 10 and 30 %) and incubation time (1, 5, 10, 20, 30, 45, 60, 75 and 90 days). All statistical analyses were performed in the SAS ver.9.2 statistical framework; to obtain the main differences between the treatments, the Duncan’s (α = 0.01) test was applied.
Results and discussion
Table 1 shows some of chemical and physical properties of applied soil. According to the sand, clay and silt contents, the soil texture was loamy sand. Table 2 presents some properties of applied plant residues. Alfalfa and wheat straw had neutral pH, high OC values and C/N and C/P ratios. Some of applied nanozeolite properties are given in Table 3. Also nanozeolite compositions and their weight percentage are shown in Table 4; according to this table, SiO2 and Al2O3 (69.44 and 11.87 %), respectively, had much higher portion than the other compositions.
The effect of nanozeolite and plant residues on OC in density fractions
Table 5 shows the analysis of variance of the effects of nanozeolite, plant residues application, incubation time and their interaction on LF, HF, soluble OC in cool water and hot water fractions in soil (p < 0.01). However, the interactions between nanozeolite and incubation time, plant residues and incubation time and nanozeolite, plant residues and incubation time did not have significant effects on mentioned OC fractions in the soil.
Table 6 reveals the OC content in LF and HF increased by the addition of nanozeolite and plant residues (p < 0.01). LF value in 30 % nanozeolite plus 5 % wheat straw (N30W5) treatment was greater than the other treatments; as its value 7.54 (g LF. kg-1 Soil) was greater than control, because C/N ratio in wheat straw was higher than alfalfa straw, and thus subsequently wheat straw had lower stage of biodegradation by microorganisms in soil. This fraction of OC decreased from the 1st day (20.61 g LF. kg-1 Soil) to the 90th day (16.99 g LF. kg-1 Soil) during soil incubation (Fig. 1). The HF value in N30A5 treatment was significantly increased (11.1 g kg-1 Soil) in comparison with the control treatment (Table 6). Also this table investigates that N30A5 treatment increased HF (3 g kg-1 Soil) and (8.2 g kg-1 Soil) in comparison with the N10A5 and N0A5 treatments, respectively.
It is known that the alfalfa straw was more efficiency due to increasing OC in HF than wheat straw in all of the treatments with the similarity percentage of nanozeolite (Table 6). SOC in the LF plays an important role in retaining of cellulase molecule from washing out and nutrition of soil microorganisms and subsequently humus production. Thus SOM quality is an important factor in its disintegration rate (Schmidt et al. 2002; Beheshti et al. 2012). According to Fig. 2, OC in HF had a distinct downward trend from the 1st day (27 g kg-1 Soil) until the 90th day (18.4 g kg-1 Soil). The recent research on OC decay dynamics showed that LF and HF were decreased during soil incubation (Hassink et al. 1995; Creamer et al. 2012; Aminiyan et al. 2016). The results of Rovira and Vallejo’ studies (2003) were in line with those of the present study.
The effect of nanozeolite and plant residues on water-soluble organic carbon fractions
As shown in Table 7, soluble OC contents in hot water and cool water increased by the addition of nanozeolite and plant residues especially alfalfa straw. The results showed that soluble OC in hot water in N30A5 treatment was greater 2.22, 1.36 and 2.06 (g kg-1 Soil) than control, N10A5 and N0A5 treatments, respectively (Table 7). Working on the chemical composition of DOC suggested that most DOC is an end product of microbial metabolism (Guggenberger and Zech 1994); However, short-term experimental manipulations of organic matter sources showed that fresh litter also contributes significantly to the production of DOC (Park et al. 2002). These two views are not necessarily mutually exclusive, but they do point out the considerable difficulty in determining the influence of substrate (litter, SOM), microbial community composition (Muller et al. 1999) and abiotic factors such as temperature and water flux on DOC production and flux (Brooks et al. 1999). Aminiyan et al. (2015) reported that the addition of 30 % nanozeolite with 5 % alfalfa straw to the soil redounded increasing OC in different aggregate particle size classes.
Based on Table 7, the same results were achieved similar to the results of hot water to cool water; accordingly, soluble OC in cool water increased with the greater percentage of nanozeolite and plant residues particularly alfalfa straw. Soluble OC in cool water value increased 1.54 and 0.58 (g kg-1 Soil) into the control in N30A5 and N30W5 treatments, respectively. Thus N30A5 treatment more effective to increasing soluble OC in cool water than N30W5 treatment. Soluble OC content in hot water was greater than soluble OC content in cool water (Table 7), because hot water had greater ability to extract of lysis microbial cells and extractable soluble organic matter may be adsorbed to clay or complexed with other organic material produced by plants or decomposing organic matter than cool water (Guggenberger and Zech 1994; Muller et al. 1999). According to the Gregorich et al. (2003) findings, high temperature is known to hydrolyze organic structures, lyse cells and dissociate organic materials from inorganic colloids. The plant residues with lower C/N ratio are a readily decomposable substrate for microorganisms, and they have additional soluble OC content than plant residues with higher C/N ratio (Gregorich et al. 2003). Also these researchers found that the quantity of biodegradable soluble organic matter was related to the extraction procedure and the quantity of organic matter present in the soil.
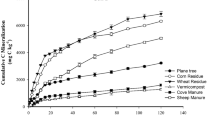
Figure 3 indicates that soluble OC in both hot water and cool water increased with over time from 1st day until the 30th day of incubation period, but then decreased by the end of the experiment. Accordingly, the soluble OC in cool water increased from 2.17 (g kg-1 Soil) in the 1st day to 2.68 (g kg-1 Soil) in the 30th day and then it decreased by the end of experiment 1.92 (g kg-1 Soil). As shown in Fig. 3, Soluble OC in hot water value increased from 2.83 (g kg-1 Soil) in the 1st day to 3.69 (g kg-1 Soil) in the 30th day, and finally it decreased by the 90th day 2.39 (g kg-1 Soil). Since soluble OC was increased with the development and promoting plant residues biodegradation in the initial 30 days and when the growth and development of microbial communities were increased with the passage of time and subsequently soluble OC decreased with the passage of time. Kalbitz et al. (2003) observed that soluble OC increased with the passage of time, but in another study soluble OC decreased by over the time (Gregorich et al. 2003). Alfalfa straw had greater soluble OC than wheat straw, and thus its degradation rate and OC content decreasing was done by higher rate in this fraction (Swanston et al. 2002; Preston and Schmidt 2006; Aminiyan et al. 2016). It is known in recent reviews that the organic matter quality is particularly important for SOC stabilization (Amelung et al. 2008; Schmidt et al. 2011).
Conclusion
Organic carbon fractions in soils play important roles in many ecosystem processes. OC fractions exhibit different rates of biochemical and microbial degradation. Density fractionation is a laboratory procedure that separates SOC into LF and HF. Also DOC is affected by the extraction procedure used. Extraction procedures involving higher temperatures extract a greater amount of soluble organic matter than extractions carried out at room temperature. The results of this study showed that LF and HF and water-soluble OC was increased by the addition of greater percentage of nanozeolite and plant residues into the soil. The results of this study showed that LF was greater in N30W5 treatment than in the other treatments. But OC in HF and soluble OC in hot and cool water had maximum amounts in (N30A5) than in the other treatments. LF and HF decreased with the passage of time from the 1st day until the 90th day. Soluble OC in hot and cool water increased from 1st day until the 30th day, and then they decreased by the end of the experiment. In fact, OC content increased by application and addition of nanozeolite and plant residues into the soil, but these pools decreased with the passage of time. Finally, it can be said that the application of nanozeolite and plant residues improve carbon sequestration process and increase carbon pools in soil.
References
Abdi GH, Khui MK, Eshghi S (2006) Effects on natural zeolite on growth and flowering on straw berry. Int J Agric Resour 1:384–389
Aitkenhead-Peterson JA, McDowell WH, Neff JC (2003) Sources, production, and regulation of allochthonous dissolved organic matter. In: Findlay S (ed) Dissolved organic matter sources, transport, and transformation in aquatic ecosystems. Academic Press, New York
Amelung W, Brodowski S, Sandhage-Hofmann A, Bol R (2008) Combining biomarker with stable isotope analyses for assessing the transformation and turnover of soil organic matter. In: Sparks DL (ed) Advances in Agronomy. Academic Press, Burlington, pp 155–250
Aminiyan MM, Sinegani AAS, Sheklabadi M (2015) Aggregation stability and organic carbon fraction in a soil amended with some plant residues, nanozeolite, and natural zeolite. Int J Recycl Org Waste Agric 4(1):11–22. doi:10.1007/s40093-014-0080-0
Aminiyan MM, Sinegani AAS, Sheklabadi M (2016) The effect of zeolite and some plant residues on soil organic carbon changes in density and soluble fractions: Incubation study. Eurasian J Soil Sci 5(1). doi:10.18393/ejss.2016.1.074-083
Balashov E, Kren J, Prochazkova B (2010) Influence of plant residue management on microbial properties and water-stable aggregates of two agricultural soils. Int Agrophys 24(1):9–14
Barrios E, Buresh RJ, Sprent JI (1996) Nitrogen mineralization in density fractions of soil organic matter from maize and legume cropping systems. Soil Biol Biochem 28:1459–1465
Beheshti A, Raiesi F, Golchin A (2012) Soil properties, C fractions and their dynamics in land use conversion from native forests to croplands in northern Iran. Agri Eco Environ 148:121–133
Bhattacharyya R, Prakash V, Kundu S, Srivastva AK, Gupta HS (2009) Soil aggregation and organic matter in a sandy clay loam soil of the Indian Himalayas under different tillage and crop regimes. Agric Ecosyst Environ 132(1):126–134
Bird M, Santruckova H, Lloyd J, Lawson E (2002) The isotopic composition of soil organic carbon on a north–south transect in western Canada. Eur J Soil Sci 53(3):393–403
Bremer E, Jansen HH, Johnston AM (1994) Sensitivity of total, light fraction and mineralizable organic matter to management practices in a Lethbridge soil. Can J Soil Sci 74:131–138
Bronick CJ, Lal R (2005) Soil structure and management: a review. Geoderma 124(1):3–22
Brooks PD, McKnight DM, Bencala KE (1999) The relationship between soil heterotrophic activity, soil dissolved organic carbon (DOC) leachate, and catchment-scale DOC export in headwater catchments. Water Resour Res 35:1895–1902
Cambardella CA, Elliott ET (1992) Particulate soil organic matter changes across a grassland sequence. Soil Sci Soc Am J 56:777–783
Cances B, Ponthieu M, Castrec-Rouelle M, Aubry E, Benedetti MF (2003) Metal ions speciation in a soil and its solution: experimental data and model results. Geoderma 113:341–355
Creamer C, Filley T, Boutton T (2012) Long-term incubations of size and density separated soil fractions to inform soil organic carbon decay dynamics. Soil Biol Biochem 51:8–11
Don A, Kalbitz K (2005) Amount and degradability of dissolved organic carbon from foliar litter at different decomposition stages. Soil Biol Biochem 37:2171–2179
Eshel G, Fine P, Singer MJ (2007) Total soil carbon and water quality: an implication for carbon sequestration. Soil Sci Soc Am J 71(2):397–405
Golchin A, Clarke P, Oades JM, Skjemstad JO (1995a) The effects of cultivation on the composition of organic matter and structural stability of soils. Aust J Soil Res 33:975–993
Golchin A, Oades JM, Skjemstad JO, Clarke P (1994) Study of free and occluded particulate organic matter in soils by solid-state 13C NMR spectroscopy and scanning electron microscopy. Aust J Soil Res 32:285–309
Golchin A, Oades JM, Skjemstad JO, Clarke P (1995b) Structure and dynamic properties of soil organic matter reflected by 13C natural abundance, pyrolysis mass spectrometry and solid-state 13C NMR spectroscopy in density fractions of an Oxisol under forest and pasture. Aust J Soil Res 33:59–76
Gregorich EG, Beare MH, Stoklas U, St-Georges P (2003) Biodegradability of soluble organic matter in maize-cropped soils. Geoderma 113:237–252
Gregorich EG, Liang BC, Mackenzie AF, Drury CF, McGill WB (2000) Elucidation of the source and turnover of water soluble and microbial biomass carbon in agricultural soils. Soil Biol Biochem 32:581–587
Guggenberger G, Zech W (1994) Composition and dynamics of dissolved organic carbohydrates and lignin degradation products in two coniferous forests, N.E. Bavaria. Germany. Soil Biol Biochem 26:19–27
Hassink J (1995) Density fractions of soil macro organic matter and microbial biomass as predictors of C and N mineralization. Soil Biol Biochem 27:1099–1108
Jansen B, Nierop KGJ, Verstraten JM (2003) Mobility of Fe(II), Fe(III) and Al in acidic forest soils mediated by dissolved organic matter: influence of solution pH and metal/organic carbon ratios. Geoderma 113:323–340
Janzen HH, Campbell CA, Brandt SA, Lafond GP, Townley-Smith L (1992) Light-fraction organic matter in soils from long-term crop rotations. Soil Sci Soc Am J 56:1799–1806
Kaiser K, Zech W (1998) Rates of dissolved organic matter release and sorption in forest soils. Soil Sci 168:714–725
Kalbitz K, Schmerwitz J, Schwesig D, Matzer E (2003) Biodegradation of soil-derived dissolved organic matter as related to its properties. Geoderma 113:273–291
Kalbitz K, Solinger S, Park JH, Michalzik B, Matzner E (2000) Controls on the dynamics of dissolved organic matter in soils: a review. Soil Sci 165:277–304
Kay BD (1998) Soil structure and organic carbon, a review. In: Lal R, Kimble JM, Follett RF, Stewart BA (eds) soil processes and the carbon cycle. CRC Press, Boca Raton, pp 169–197
Kiikkilä O, Kanerva S, Kitunen V, Smolander A (2014) Soil microbial activity in relation to dissolved organic matter properties under different tree species. Plant Soil 377(1–2):169–177
Kim Y, Ullah S, Moore TR, Roulet NT (2014) Dissolved organic carbon and total dissolved nitrogen production by boreal soils and litter: the role of flooding, oxygen concentration, and temperature. Biogeochemistry 118(1–3):35–48
Liang Q, Chen H, Gong Y, Fan M, Yang H, Lal R, Kuzyakov Y (2012) Effects of 15 years of manure and inorganic fertilizers on soil organic carbon fractions in a wheat-maize system in the North China Plain. Nutr Cycl Agroecosyst 92(1):21–33
Magid J, Gorissen A, Giller KE (1996) In search of the elusive ‘‘active’’ fraction of soil organic carbon: three size-density fractionation methods for tracing the fate of homogeneously 14C labeled plant materials. Soil Biol Biochem 28:89–99
Mahmoodabadi M, Heydarpour E (2014) Sequestration of organic carbon influenced by the application of straw residue and farmyard manure in two different soils. Int Agrophys 28(2):169–176
Majumder B, Mandal B, Bandyopadhyay PK (2008) Soil organic carbon pools and productivity in relation to nutrient management in a 20-year-old rice–berseem agroecosystem. Biol Fertil Soils 44(3):451–461
Marschner B, Kalbitz K (2003) Controls of bioavailability and biodegradability of dissolved organic matter in soils. Geoderma 113:211–235
Miller RM, Jastrow JD (1990) Hierarchy of root and mycorrhizal fungal interactions with soil aggregation. Soil Biol Biochem 22:579–584
Muller J, Miller M, Kjuller A (1999) Fungal–bacterial interaction on beech leaves: influence on decomposition and dissolved organic carbon quality. Soil Biol Biochem 31:367–374
Park JH, Kalbitz K, Matzner E (2002) Resource control on the production of dissolved organic carbon and nitrogen in a deciduous forest floor. Soil Biol Biochem 34:813–822
Preston CM, Schmidt MWI (2006) Black (pyrogenic) carbon: a synthesis of current knowledge and uncertainties with special consideration of boreal regions. Biogeosciences 3:397–420
Rovira P, Ramón Vallejo V (2003) Physical protection and biochemical quality of organic matter in Mediterranean calcareous forest soils: a density fractionation approach. Soil Biol Biochem 35:245–261
Sanderman J, Baldock JA, Amundson R (2008) Dissolved organic carbon chemistry and dynamics in contrasting forest and grassland soils. Biogeochemistry 89:181–198
Schmidt MWI, Skjemstad JO, Jager C (2002) Carbon isotope geochemistry and nanomorphology of soil black carbon: black chernozemic soils in central Europe originate from ancient biomass burning. Global Biogeochem Cycles 16(4):1–70
Schmidt MWI, Torn MS, Abiven S, Dittmar T (2011) Persistence of soil organic matter as an ecosystem property. Nature 478:49–56
Six J, Callewaert P, Lenders S, De Gryze S (2002) Measuring and understanding carbon storage in afforested soils by physical fractionation. Soil Science Society of American Journal 66:1981–1987
Smolander A, Kitunen V (2011) Comparison of tree species effects on microbial C and N transformations and dissolved organic matter properties in the organic layer of boreal forests. Appl Soil Ecol 49:224–233
Sollins P, Glassman C, Paul EA, Swanston C, Lajtha K, Heil JW, Elliott EA (1999) Soil carbon and nitrogen: pools and fractions. In: Robertson GP, Bledso CS, Coleman C, Sollins P (eds) Standard soil methods for long-term ecological research. Oxford University Press, New York, pp 89–105
Stevenson FJ (1994) Humus chemistry: genesis, composition, reaction, 2nd edn. Wiley, New York, p 496
Straathof AL, Chincarini R, Comans RN, Hoffland E (2014) Dynamics of soil dissolved organic carbon pools reveal both hydrophobic and hydrophilic compounds sustain microbial respiration. Soil Biol Biochem 79:109–116
Strobel BW, Hansen HCB, Borggaard OK, Andersen MK, Raulund-Rasmussen K (2001) Composition and reactivity of DOC in forest floor soil solutions in relation to tree species and soil type. Biogeochemistry 56:1–26
Swanston CW, Caldwell BA, Homann PS, Ganio L, Sollins P (2002) Carbon dynamics during a long-term incubation of separate and recombined density fractions from seven forest soils. Soil Biol Biochem 34:1121–1130
Tan Z, Lal R, Owens L, Izaurralde RC (2007) Distribution of light and heavy fractions of soil organic carbon as related to land use and tillage practice. Soil Tillage Res 92:53–59
Thelen KD, Fronning BE, Kravchenko A, Min DH, Robertson GP (2010) Integrating livestock manure with a corn–soybean bioenergy cropping system improves short-term carbon sequestration rates and net global warming potential. Biomass Bioenergy 34(7):960–966
Walkley A, Black IA (1934) An examination of digestion method for determining soil organic matter and a proposed modification of the chromic acid titration. Soil Sci 37:29–38
Wander MM, Traina SJ (1996) Organic matter fractions from organically and conventionally managed soils. 1. Carbon and nitrogen distribution. Soil Sci Soc Am J 60:1081–1087
Wang X, Cammeraat LH, Wang Z, Zhou J, Govers G, Kalbitz K (2013) Stability of organic matter in soils of the Belgian Loess Belt upon erosion and deposition. Eur J Soil Sci 64:219–228
Wang Y, Xu H, Wu X, Zhu Y, Gu B, Niu X, Liu A, Peng Ch, Ge Y, Chang J (2011) Quantification of net carbon flux from plastic greenhouse vegetable cultivation: a full carbon cycle analysis. Environ Pollut 159(5):1427–1434
Yano Y, McDowell WH, Aber JD (2000) Biodegradable dissolved organic carbon in forest soil solution and effects of chronic nitrogen deposition. Soil Biol Biochem 32:1743–1751
Zhao M, Zhou J, Kalbitz K (2008) Carbon mineralization and properties of water extractable carbon in soils of the south Loess Plateau in China. Eur J Soil Biol 44:158–165
Zsolnay A, Gorlitz H (1994) Water-extractable organic matter in arable soils: effects of drought and long-term fertilization. Soil Biol Biochem 26:1257–1261
Acknowledgments
The authors sincerely thank editor and the anonymous reviewers for reviewing the manuscript and providing critical comments to improve the paper. Thanks so much for Fadak institute in Isfahan and Afrazand factory in Tehran.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Aminiyan, M.M., Sinegani, A.A.S. & Sheklabadi, M. Assessment of changes in different fractions of the organic carbon in a soil amended by nanozeolite and some plant residues: incubation study. Int J Recycl Org Waste Agricult 4, 239–247 (2015). https://doi.org/10.1007/s40093-015-0110-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40093-015-0110-6