Abstract
Background
Water hyacinth (Eichhornia crassipes) can cause a diversity of problems due to its fast spread and congested growth. The composting of water hyacinth can reduce the chemical fertilizer application to the agricultural field and problems related to its fast growth rate and also help in nutrient recycling. The present study was carried out on agitated pile composting of water hyacinth collected from four different areas (Bharalu River, Agriculture site, Boragaon landfill site and Industrial site). The nutrients and stability parameters were evaluated during the 30 days of agitated pile composting of water hyacinth mixed with cattle manure and sawdust. The stability of compost was evaluated using respiration techniques (CO2 evolution and oxygen uptake rate).
Results
Results showed that nutrients (Na, K, Ca, total nitrogen and phosphorus) were increased significantly in all agitated pile composting. Stability parameters such as CO2 evolution rate and oxygen uptake rate, biochemical chemical oxygen demand and chemical oxygen demand were reduced significantly during the process. Highest reduction of moisture content (35.2 %), volatile solid (40.5 %), ammonical nitrogen (75.6 %), biochemical oxygen demand (65.3 %), CO2 evolution rate (78.7 %), and oxygen uptake rate (74.4 %) were observed in the agitated pile of Bharalu River site as compared to other agitated piles. The higher increase of total nitrogen (83.5 %) and total phosphorus (76.0 %) was also observed in the agitated pile of Bharalu River site.
Conclusion
Addition of optimum amount of cattle manure was highly efficient for making compost of water hyacinth collected from four different sites. Highest content of nutrients was found in compost of water hyacinth collected from Bharalu River site due to availability of nutrients in sewage water. In the final compost of all trials, total coliform and fecal coliform were reduced significantly, which are pathogen indicators in the compost. The best compost quality was found in water hyacinth collected from Bharalu River site.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Water hyacinth (Eichhornia crassipes) is a free-floating macrophyte, growing in or near water, that is emergent, submerged or free floating (Singh and Kalamdhad 2012). Due to very high productivity, water hyacinth has been considered as one of the worst aquatic plants of the world. It can double its size in 5 days under optimum temperature and moisture. The dense mats of water hyacinth can interfere with navigation, recreation and irrigation as well as the blockage of canals and rivers can even cause dangerous flooding. Availability of oxygen is low beneath the mats that generate good breeding conditions for mosquito vectors of malaria, encephalitis and filariasis (Gajalakshmi et al. 2002; Malik 2007). Composting is the most promising technique for the treatment of water hyacinth; composting can solve two problems: (a) reduce chemical fertilizer load and (b) check the abnormal growth of hyacinth. The formation of humic substances is the final product of any type of composting process; it holds sufficient quantity of nutrients (nitrogen, potassium, calcium and phosphorus) which are required for the plants growth Gajalakshmi et al. 2002; Prasad et al. 2013; Singh and Kalamdhad 2013a). Water hyacinth compost application in agriculture mainly results from its content of organic matter, plant nutrients, promoting plant growth and inhibiting root pathogens/soil-borne plant diseases (Balasubramanian et al. 2013).
As the composting process proceeds, the readily degradable organic mass is converted into stable humic substances (Haug 1993). The compost is considered stable if it contains mainly recalcitrant or humus-like matter, but some times unstable when it contains a high quantity of biodegradable matter that may maintain high microbial activity (Kalamdhad et al. 2009). Stability of compost is a key parameter in relation to its field application, potential of odor generation and pathogen regrowth. The stability of composts can be defined as the degree to which the organic fractions in composts have been stabilized during the process (Kalamdhad et al. 2009).
A Boragaon landfill (Guwahati, India) may cause contamination of soil and water via leaching. This landfill has covered the surrounding area of the Deepor Beel Lake. Leachate is generated by landfill possibly entering into lake and pollutes fresh water resource. Growing water hyacinth in those areas may accumulate nutrient from the polluted water. Bharalu River is highly polluted by sewage of Guwahati city, India. Water hyacinth plants are growing in this River accumulating high concentration of nutrients. Loss of nutrient from agriculture field due to runoff in low-lying areas is very common phenomenon in the agricultural field. These are the most favorable conditions for the growth of the water hyacinth which are often found in developing countries. Rising water hyacinth in these sites may contain high concentration of nutrients. Some studies have been done on agitated pile composting of different wastes (Parkinson et al. 2004; Jouraiphy et al. 2005; Singh and Kalamdhad 2012, 2013a, b), but there is no information available on composting of water hyacinth collected from different sources. Therefore, the objective of the present study was to assess the nutrients and stability parameters during agitated pile composting of water hyacinth collected from Bharalu River site (BRS), agriculture site (AS), boragaon landfill site (BLS) and industrial site (IS).
Materials and methods
Feedstock materials and design of agitated pile composting
The composting study was carried out in the summer season (from June, 2013 to July, 2013). Water hyacinth, cattle (cow) manure and sawdust were used for the preparation of different waste mixtures for the composting process. Water hyacinth was collected from four different places, i.e. Amingoan industrial site (IS) near Indian Institute of Technology Guwahati (IITG) campus, Boragaon landfill site (BLS), Bharalu River site (BRS) and low-lying area near agriculture site (AS), Guwahati, India. Water hyacinth (shoot + root) was collected from 4 to 5 different places at each sampling site to ensure the grab sampling. All collected materials were processed in the laboratory before composting process. The maximum particle size of water hyacinth in the waste mixture was restricted to 1 cm by chopping to provide better aeration. Cattle manure was obtained from dairy farm near the IITG campus. Sawdust was purchased from nearby saw mill. On the basis of our previous studies (Singh and Kalamdhad 2012, 2013a, b; Prasad et al. 2013), a mixture of 90 kg water hyacinth, 45 kg cattle manure and 15 kg sawdust (6:3:1 ratio of water hyacinth, cattle manure and sawdust) was taken for the study. This was the best combination for nutrient recycling and stability analysis during water hyacinth composting; therefore, in this study same combinations have been used with water hyacinth collected from four different sources. The initial characterizations of raw materials are given in Table 1. Four different waste combinations were composted in agitated pile (length 2100 mm, base width 350 mm, top width 100 mm and height 250 mm). A length to base width (L/W) ratio of 6 was maintained for each agitated pile. Agitated piles contained approximately 150 kg of different waste combinations and composted for 30 days. Temperature was monitored by a digital thermometer during the composting period. About 500 g of homogenized samples were collected from five different locations, mostly at the mid span and ends of the pile by compost sampler without disturbing the adjacent materials in the pile on days 0, 3, 6, 9, 12, 15, 18, 21, 24, 27, and 30 after turning. Finally all the grab samples were mixed carefully to make a homogenized sample. Triplicate samples were collected and stored at 4 °C for analysis of biochemical parameters. Sub-samples were dried at 105 °C in oven for 24 h and moisture content was calculated as given in Eq. 1, Dried samples were ground to pass to 0.2 mm sieves and stored for further analysis.
Analysis of physico-chemical and biological parameters
Each sub-sample was analyzed for the following parameters: pH and electrical conductivity (EC) (1:10 w/v waste: water extract, and volatile solids (VS) (Kalamdhad et al. 2009). Volatile solid was determined by loss ignition method; 10 g sample was taken in crucible and kept in muffle furnace at 550 °C for 2 h. The total nitrogen (TN) was determined using the Kjeldahl method; 0.2 g of sample (dried) was digested with 10 ml of concentrated H2SO4 and catalyst mixture (CuSO4:K2SO4, 5:1 ratio) and heated at 400 °C. After digestion, sample was diluted to 100 mL with distilled water. 10 mL of diluted sample was mixed with 20 mL of 40 % NaOH and subjected to the distillation assembly. Ammonia gas was collected in 25 mL of boric acid solution in 250 mL conical flask and then titrated with standardized H2SO4 (0.02 N). Boric acid solution was prepared with mixed indicator (methyl red and methylene blue). The end point is the appearance of purple colour. NH4–N and NO3–N were determined using KCl extraction (Tiquia and Tam 2000); a 3-g oven-dried sample was taken in a 250 mL conical flask and mixed with 30 mL 2 M KCl solution. The mixture was placed in a horizontal shaker for 2 h. The analysis for NH4 +–N in the filtered sample was done using Phenate Method (APHA 2005). The Flame photometer (Systronic 128) was used for analysis of Na, K and Ca concentration after digestion of the 0.2-g sample with 10 mL H2SO4 and HClO4 mixture (5:1) in block digestion system (Pelican Equipments Chennai-India) for 2 h at 300 °C. Above acid-digested sample was also taken for the determination of available and total phosphorus (acid digestion) using the stannous chloride method (APHA 2005).Calculations of VS and TN of compost samples are given in Eq. 2 and 3, as follows:
where S mL of standard sulfuric acid used for sample, B mL of standard sulfuric acid used for blank, N normality of standard sulfuric acid
The biodegradable organic matter was measured as soluble biochemical oxygen demand (BOD) (by the dilution method; APHA 2005) and soluble chemical oxygen demand (COD) (by the dichromate method; APHA 2005). The population of total coliforms (TC) in the compost was determined in extract (compost:water extract, 1:10) by inoculation of culture tube media using the most probable number (MPN) method (APHA 2005).
The stability parameters, i.e. oxygen uptake rate (OUR) and CO2 evolution rate were determined according to the Kalamdhad et al. (2008).For the determination of CO2 evolution rate, about 25 g of fresh compost sample was taken in 1 L of polyvinyl chloride airtight container. About 10 g of oven-dried (105 °C) soda lime (1.5–2.0 mm mesh size) was taken in a 100-mL beaker and placed in the above container. The initial weight of the soda lime was measured. The container with soda-lime beaker was kept in an incubator at 25 °C temperature for 24 h. After 24 h, the soda-lime was taken out and oven dried again; then the final weight was noted down.CO2 evolution rate was calculated by the following formula Eq. (4) (Kalamdhad et al. 2008):
where W1 is initial weight of the soda-lime (g), W2 is final weight of the soda-lime (g), W is weight of compost sample (g) and T is time duration of incubation (h).
The OUR was measured in a liquid suspension of 5–8 g of compost sample in 500 mL of distilled water in which CaCl2, MgSO4, FeCl3 and phosphate buffer (pH 7.2) were added. The mixture was kept in suspension by placing it on the magnetic stirrer at 30°C temperature. During this time, the dissolved O2 in the suspension was continuously measured by the digital dissolved oxygen meter attached. The oxygen consumption rate was calculated by taking the difference of dissolved oxygen with the respect to the time intervals and calculated value was quoted as the OUR in mg O2/g VS/h (Kalamdhad et al. 2008).
All the results reported are the means of three replicates. Repeated measures treated with analysis of variance (ANOVA) was made using SPSS software. The objective of the statistical analysis was to determine any significant difference among the parameters for different agitated piles.
Results and discussion
Physico-chemical analysis
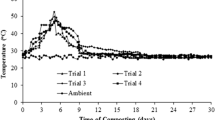
The composting pile temperature went through three typical phases (mesophilic, thermophilic and cooling phase) and ranged from 22 to 52 °C during the entire period of composting (Fig. 1). The cattle manure affected the temperature during the agitated pile composting in all four different agitated piles. Addition of optimum amount of cattle manure to the composting materials may fasten the composting process by providing easily available carbon to the microorganisms present in the composting mass. Therefore, microbial metabolic activities have increased and temperature was increased in very short time (Singh and Kalamdhad 2012, 2014). In agitated pile composting of BRS reached the highest temperature among all four piles due to quick establishment of microbial activity during the composting process (Chen et al. 2010). Although addition of cattle manure increased the composting process in all agitated piles the results indicate that the composting microbes started utilizing the easily degradable materials at the earliest in BRS. The highest temperature was observed in BRS (52 °C) followed by BLS (51.3 °C), AS (50.8 °C) and IS (48.5 °C). After 12th day, temperature in all piles was reduced and reached near-ambient temperature. Reduction in temperature indicated all piles achieved stability within 30 days of composting period.
Initial composting mixture requires optimum moisture content for the survival of microorganisms. The reduction in moisture content during the composting process in the form of vapors by the heat generation can be viewed as an index of decomposition rate (Kalamdhad et al. 2009). Initial moisture contents were observed about 83.4, 80.9, 82.9 and 82.2 % which were reduced to 54.1, 57.7, 54.3 and 59.9 % during pile composting of water hyacinth collected from BRS, AS, BLS and IS, respectively (Fig. 2a). Highest moisture loss occurred in agitated pile of BRS (35.2 %) followed by BLS (34.5 %), AS (28.6 %) and IS (27.1 %). On analyzing the results by ANOVA, the decrease in moisture content varied significantly between the days (P < 0.05).
Figure 2b illustrates that the pH was increased from 6.5 to 7.5, 6.2 to 7.2, 6.4 to 7.2 and 7.1 to 7.8 in agitated pile of BRS, IS, BLS and IS, respectively. The pH values were increased during the composting process may be due to sufficient aeration provided through the turning of piles at every 3rd day. Consequently, carbon dioxide level was reduced during the composting process (Singh and Kalamdhad 2013a). Significant difference in pH was observed in all the trials (P < 0.05). The pH values were measured within the optimal range for the development of bacteria (6.0–7.5) and fungi (5.5–8.0) (Amir et al. 2005).
Electrical conductivity (EC) is generally measured during composting because it reflects the salinity of the compost and its appropriateness for plant growth (Singh and Kalamdhad 2013a).Fig. 2c depicts the reduction of EC from 8.1 to 6.4 dS/m, 5.7 to 3.7 dS/m and 7.7 to 5.2 dS/m in the agitated pile of BRS, IS and BLS, respectively; however, EC was increased about 2.2–3.2 dS/m in agitated pile of IS. EC was decreased during the composting process probably due to volatilization of ammonia and the precipitation of mineral salts (Kalamdhad et al. 2009). In the agitated pile of IS, EC was enhanced probably due to the net loss of weight and release of soluble salts through decomposition activity in the composting process. On analyzing the results by ANOVA, EC varied significantly between the all agitated piles (P < 0.05).
Figure 2d shows the trend of organic matter degradation during the 30-day composting process in four different agitated piles. The content of organic matter was decreased from 61.7, 58.0, 61.4 and 53.7 % to 36.7, 40.2, 40.1 and 38.9 % in the agitated pile of BRS, AS, BLS and IS, respectively, as the decomposition progressed. Higher loss of organic matter was observed in agitated pile of BRS (40.5 %) followed by BLS (34.8 %), AS (30.6 %) and IS (27.6 %). BRS composting shows higher loss of organic matter as a result of higher temperature evolution as compared to other agitated piles. A significant variation in organic matter loss was found in all the agitated piles (P < 0.05). In the composting process organic matter is decomposed and transformed to stable humic substances (Prasad et al. 2013).
Table 2 shows that the total nitrogen was increased significantly (P < 0.05) from 0.85, 0.83, 1.05 and 0.64 % to 1.56, 1.19, 1.47 and 1.05 % in the agitated pile of BRS, AS, BLS and IS, respectively, during the composting process. The maximum increase in total nitrogen was observed in agitated pile of BRS (83.5 %) followed by IS (64.1 %), AS (43.4 %) and BLS (40.0 %). The total nitrogen was increased from 0.9 to 1.6 %, from 0.8 to 1.2 %, from 1.1 to 1.5 % and from 0.6 to 1.1 % in BRS, AS, BLS and IS, respectively, within the composting time. It might be due to the net loss of dry mass in terms of CO2 as well as the water loss by evaporation during oxidization of organic matter (Prasad et al. 2013). The total nitrogen generally increases during composting when organic matter loss is greater than the loss of NH4–N (Sarika et al. 2014). The ammonical nitrogen was reduced significantly (P < 0.05) from 253.5, 42.3, 223.3 and 70.7 mg/kg to 61.9, 21.5, 94.3 and 34.8 mg/kg in the agitated pile of BRS, AS, BLS and IS, respectively (Table 2). The maximum reduction of ammonical nitrogen was found in agitated pile of BRS (75.6 %) followed by BLS (57.8 %), IS (50.7 %) and IS (49.1 %) during the process. At high pH, mixing and proper aeration enhance ammonia loss during maturation (Kalamdhad et al. 2009). Table 2 demonstrates that the total and available phosphorus were increased significantly (P < 0.05) in all agitated piles. The total phosphorus concentration was increased from 3.1, 1.7, 3.3, and 2.2 g/kg to 5.4, 2.8, 5.5 and 3.4 g/kg in BRS, AS, BLS and IS, respectively. The available phosphorus concentration was increased from 2.8 to 3.6 mg/kg, from 1.7 to 2.2 mg/kg, from 1.6 to 1.9 mg/kg and from 1.4 to 2.4 g/kg in BRS, AS, BLS and IS, respectively, in the 30 days of composting process. The concentrations of total and available phosphorus were increased during the composting process might be due to phosphorus released by micro-organisms through the mineralization of organic matter. The results of present study were consistent with those of the other study (Sarika et al. 2014). The highest percentage of total phosphorus and available were increased in the compost of BRS (76.0 %) and IS (66.3 %), respectively. Figure 3 shows that nutrient (Na, K and Ca) concentration was increased significantly (P < 0.05) due to degradation of organic matter during the composting process (Huang et al. 2004). The concentrations of nutrients (Na, K and Ca) were increased in the range of 1.21–6.89 g/kg for Na, 18.68–67.43 g/kg for K and 5.18–14.88 g/kg for Ca in final compost of all agitated piles. Nutrient concentrations were increased during composting process due to the net loss of dry mass (Sarika et al. 2014).
Biological analysis
Respiration of organic matter during degradation is measured as BOD, COD, OUR and CO2 evolution rate. These parameters reduced with organic matter degradation, consequentially decreased the emission of carbon dioxide, ultimately indicating stabilization of the compost (Kalamdhad and Kazmi 2009). Figure 4a, b demonstrates that the soluble BOD and COD were reduced significantly (P < 0.05). The higher reduction in BOD was found in BRS (65.3 %) followed by BLS (63.3 %), AS (61.6 %) and IS (59.3 %); however, higher reduction in COD was found in IS (77.5 %) followed by BRS (69.7 %), BLS (63.2 %) and AS (62.3 %).
The CO2 evolution directly correlates to aerobic respiration, the measurement of respiration and consequently aerobic biological activity (Kalamdhad et al. 2009). The OUR is the most accepted method for the determination of biological activity of a materials; it measures compost stability by assessing the amount of easily biodegradable organic matter still present in the compost through its carbonaceous oxygen demand (Singh et al. 2009). Significantly higher reduction (P < 0.05) in CO2 evolution rate was found in BRS (78.7 %) followed by IS (77.0 %), BLS (67.8 %) and AS (66.8 %) (Fig. 4c), while higher reduction in OUR was found in BRS (74.4 %) followed by AS (71.2 %), BLS (71.1 %) and IS (68.6 %) during the process (P < 0.05) (Fig. 4d). The presence of coliform bacteria is generally used as an indicator of overall sanitary quality of the compost. Most of the pathogens and parasites are destroyed at high temperatures during the composting process. The fecal coliform was within the recommended limit of density 5 × 102 MPN/g dry weights for the compost hygienization (Kalamdhad et al. 2009). The total coliform levels declined from 2.3 × 1012 to 5.3 × 102, 1.5 × 108 to 2.1 × 106, 9.3 × 1010 to 4.6 × 107 and 1.1 × 1010 to 1.5 × 106 MPN/g wet weight in the agitated pile of BRL, AS, BLS and IS, respectively. The fecal coliform levels were also reduced from 3.6 × 108 to 23, 4.3 × 102 to 28, 3.6 × 102 to 1.2 × 102 and 2.4 × 102 to 15 MPN/g wet weight in the agitated pile of BRL, AS, BLS and IS, respectively, during the composting process. The results of present study were consistent with the results reported by Prasad et al. (2013) during the agitated pile composting of water hyacinth performed with different ratios of cattle manure, sawdust and water hyacinth.
The reduction of total and fecal coliform can be attributed to pathogens killed at high temperatures during thermophillic phase (Hassen et al. 2001)
Conclusion
Addition of optimum amount of cattle manure provides easily available carbon sources for composting microorganisms. The pH was changed from slightly acidic to neutral at the end of composting process. Highest reduction of moisture content, organic matter, ammonical nitrogen, CO2 evolution rate and oxygen uptake rate was observed in the agitated pile of Bharalu River site as compared to industrial site, agricultural site and Boragaon landfill site during the composting process. The nutrient (nitrogen, available phosphorus, Na, K and Ca) content was increased significantly in the final compost of all agitated piles but higher increment was found in the final compost of water hyacinth collected from Bharalu River site. Total and fecal coliform were reduced significantly, which are pathogen indicators in the compost. Therefore, the present study concluded that the addition of optimum amount of cattle manure was very efficient for composting water hyacinth collected from four different sites, but better compost quality was found in water hyacinth collected from Bharalu River site.
References
Amir S, Hafidi M, Merlina G, Revel JC (2005) Sequential extraction of heavy metals during composting of sewage sludge. Chemosphere 59:801–810
APHA (2005) Standard methods for the examination of water and wastewater, 21st edn. American Public Health Association, Washington
Balasubramanian D, Arunachalam K, Arunachalam A, Das AK (2013) Water hyacinth [Eichhornia crassipes (Mart.) Solms.] engineered soil nutrient availability in a low-land rain-fed rice farming system of north-east India. Ecol Eng 58:3–12
Chen YX, Huang XD, Han ZY, Huang X, Hu B, Shi DZ, Wu WX (2010) Effects of bamboo charcoal and bamboo vinegar on nitrogen conservation and heavy metals immobility during pig manure composting. Chemosphere 78:1177–1181
Gajalakshmi S, Ramasamy EV, Abbasi SA (2002) High-rate composting–vermicomposting of water hyacinth (Eichhornia crassipes, Mart. Solms). Bioresour Technol 83:235–239
Hassen A, Belguith K, Jedidi N, Cherif A, Cherif M, Boudabous A (2001) Microbial characterization during composting of municipal solid waste. Bioresour Technol 80(217):225
Haug RT (1993) The practical handbook of composting engineering. Lewis publishers Boca Raton, Landon
Huang GF, Wong JWC, We QT, Nagar BB (2004) Effect of C/N on composting of pig manure with sawdust. Waste Manage 24:805–813
Jouraiphy A, Amir S, El Gharous M, Revel J-C, Hafidi M (2005) Chemical and spectroscopic analysis of organic matter transformation during composting of sewage sludge and green plant waste. Int Biodeterior Biodegrad 56:101–108
Kalamdhad AS, Kazmi AA (2009) Effects of turning frequency on compost stability and some chemical characteristics in a rotary drum composter. Chemosphere 74:1327–1334
Kalamdhad AS, Pasha M, Kazmi AA (2008) Stability evaluation of compost by respiration techniques in a rotary drum composter. Resour Conserv Recy 52:829–834
Kalamdhad AS, Singh YK, Ali M, Khwairakpam M, Kazmi AA (2009) Rotary drum composting of vegetable waste and tree leaves. Bioresour Technol 100:6442–6450
Malik A (2007) Environmental challenge vis a vis opportunity: the case of water hyacinth. Environ Int 33:122–138
Parkinson R, Gibbs P, Burchett S, Misselbrook T (2004) Effect of turning regime and seasonal whether conditions on nitrogen and phosphorus losses during aerobic composting of cattle manure. Bioresour Technol 91:171–178
Prasad R, Singh J, Kalamdhad AS (2013) Assessment of nutrients and stability parameters during composting of water hyacinth mixed with cattle manure and sawdust. Res J Chem Sci 3(4):1–4
Sarika D, Prasad R, Singh J, Vishan I, Varma VS, Kalamdhad AS (2014) Study of physico-chemical and biochemical parameters during rotary drum composting of water hyacinth. Int J Recycl Org Waste Agric 3(63):1–10
Singh J, Kalamdhad AS (2012) Concentration and speciation of heavy metals during water hyacinth composting. Bioresour Technol 124:169–179
Singh J, Kalamdhad AS (2013a) Assessment of bioavailability and leachability of heavy metals during rotary drum composting of green waste (Water hyacinth). Ecol Eng 52:59–69
Singh J, Kalamdhad AS (2013b) Effect of lime onbioavailability and leachability of heavy metals during agitated pile composting of water hyacinth. Bioresour Technol 138:148–155
Singh J, Kalamdhad AS (2014) Effects of natural zeolite on speciation of heavy metals during agitated pile composting of water hyacinth. Int J Recycl Org Waste Agricult 3(55):1–17
Singh YK, Kalamdhad AS, Ali M, Kazmi AA (2009) Maturation of primary stabilized compost from rotary drum composter. Resour Conserv Recyc 53:386–392
Tiquia SM, Tam NFY (2000) Fate of nitrogen during composting of chicken litter. Environ Pollut 110:535–541
Acknowledgments
The authors gratefully acknowledge the financial support of the Department of Science and Technology (DST), Government of India.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Singh, J., Kalamdhad, A.S. Assessment of compost quality in agitated pile composting of water hyacinth collected from different sources. Int J Recycl Org Waste Agricult 4, 175–183 (2015). https://doi.org/10.1007/s40093-015-0097-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40093-015-0097-z