Abstract
Background
In India, fishmeal is habitually considered as the major protein source for fish culture. Rising cost, deteriorating quality and unavailability of fishmeal caused huge difficulty in modern aquaculture, particularly for fish nutrition. Nearly all Anabas testudineus cultures faced loss, due to high feed costs, inappropriate feed formulation and management. So it was necessary to find a profitable replacement of fishmeal, which could provide better growth performance to A. testudineus.
Results
In this study, different types of poultry wastes were tested, and poultry viscera confirmed satisfactory results, having 60.67 % crude protein (% Dry matter basis). Further, a feeding trial was conducted for 60 days in 90-L circular fibre tanks with proper aeration, to evaluate poultry viscera, in the formulated diet for A. testudineus. Triplicate groups of fingerlings each were fed four isonitrogenous diets, at 5 % of wet body weight basis. Feed readjusted biweekly. Comparatively, fish accumulated highest and significant (P < 0.05) increase in body weight and fat deposition, when dietary fishmeal completely replaced by poultry viscera.
Discussions
Thus, this study revealed that better growth performance in Koi, A. testudineus (Bloch) could be achieved through utilization of poultry viscera in the formulated fish feed, compared to fishmeal. Hence, poultry waste recycling could be stimulated also.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fish as the prime animal protein source in the nutritional budgeting of West Bengal (Eastern India) deliver high protein, low cholesterol and tasty-soft flesh, etc. About 85–90 % fish protein is digestible and all the dietary essential amino acid is in fish flesh (Cappell et al. 2007).
Climbing Perch (Anabas testudineus) is locally known as ‘Koi’ in West Bengal. It is one of the most popular fishes in West Bengal, due to its traditional role as a medically prescribed diet for sick and convalescents. It contains high amount of available iron and copper essentially needed for haemoglobin synthesis. In addition, it also contains all the essential amino acids (Saha 1971).
Anabas testudineus encompasses a wide range of geographical distribution. This species is also found in India, Pakistan, Burma, Sri Lanka, Thailand, China, Hong Kong, Philippines and Malaysia (Jayaram 1981). It is found in fresh and brackish waters mostly in ponds, swamps and lakes. Climbing perch appears to be visual feeders, feeding primarily during the day (Patra 1993). It is a ferocious predator, omnivorous in feeding habit and tolerate wide range of environmental conditions.
In adverse atmospheric circumstances, this fish proves to be a very good challenger of fish culture. But, often its production fails to fulfil its high market demand, due to lack of proper culture. However, the results of the survey in 2002, more than 50 % climbing perch culture farmers were lost due to high feed costs (Tihn 2003).
There are very little information available based on the nutritional requirement of A. testudineus. It is important to know the minimum protein requirement for best growth in formulating a balanced diet as protein is the key nutrient offering growth and other metabolic activities.
Usage of combination of various animal or plant protein ingredients in the place of fish meal in fish diet has been demonstrated successfully (Millamena 2002). Viscera are the large organs inside the body of animals, such as the heart, lungs and stomach, etc. Research findings have revealed that Recycling of wastes from poultry slaughter houses is of economical, biological and environmental importance (Cai et al. 1994; Steffens 1994). However, poultry wastes recycling could be stimulated by the utilization of poultry viscera.
Considering the above, the present study was conducted with a view to set up the best dietary protein requirement of A. testudineus and to assess the result of replacing dietary fishmeal protein by poultry viscera, on the growth performance of A. testudineus.
Materials and methods
Experimental sites and study period
During the study period, from April, 2012 to May, 2013 fish feed ingredients were collected and then transported to the Zoology Laboratory, Visva-Bharati, Birbhum, W.B, India (23°41′30″N Latitude and 87°41″20E Longitude).
Collection and storage of samples
Samples of feed ingredients such as Poultry viscera, fish meal, rice bran, wheat bran, Mustard oil cakes, wheat flour, etc., were collected, and sun dried properly, then packed in polyethylene bags to prevent initial spoilage and brought to the ASEPAN laboratory Visva-Bharati, then stored in refrigerator.
Proximate composition analysis
Proximate analysis is usually the first step in the chemical evaluation of a feed ingredient, where the material is subjected to a series of relatively simple chemical tests so as to determine the content of moisture, crude protein, lipid, crude fibre, ash, etc.
Estimation of moisture (%)
Moisture is commonly determined by drying a sample at some elevated temperature (100 ± 5 °C) for 30 min and further at 60 °C, until a constant weight was obtained, following [Association of Official Analytical Chemicals (AOAC 1995)].
Determination of ash (%)
Ash is readily determined by ignition from dried sample at about 550 ± 50 °C for 6–8 h in muffle furnace. The residue is weighed and reported as ash, following AOAC (1995).
Determination of crude protein (%)
The crude protein determined by Micro-Kjeldahl method using a Kjeltec system (Tecator, Sweden) through digestion and distillation steps. Kjeltab (containing potassium sulphate and catalysts), H2SO4, NaOH, Phenolphthalein, etc., used in this method, following Pearson (1999).
Determination of crude lipid (%)
The lipid content was determined by Soxhlet apparatus. 2gm of sample wrapped in whatman filter paper (No-1) and placed in a thimble connected with Soxhlet apparatus. Initial weight of soxhlet flask recorded and filled with 200 ml petroleum ether, which boiled for 8 h at 60–80 °C through the thimble, by siphoning process. Flask was taken out and allowed to evaporate. The difference in the two weights of the round joint flask gave the weight of the lipid, following AOAC (1995).
Analysis of fibre (%)
Done by following the method of Pearson (1976).
Nitrogen-free extract (%)
{100 − (% moisture + % crude protein + % crude lipid + % crude fibre + % ash)} following AOAC (1995).
Carbohydrate (%)
[Nitrogen-free extract % + Crude Fibre %] following Hastings (1976).
Caloric value/gross energy (kcal/100 g)
[(Carbohydrate × 4.1/100) + (Protein × 5.65/100) + (Lipid × 9.45/100)]. After (Cho et al. 1982).
Growth parameters
The following parameters were used to evaluate the growth, by using the following formulas according to Castell and Tiews (1980):
Survival rate (%) = 100 × (final number of fish/initial number of fish).
Specific growth rate (SGR) (%/day) = 100 x (Ln Wf − Ln Wi)/Δt.
Feed conversion ratio (FCR) = feed consumed/(Wf − Wi). Where: Wf = final weight and Wi = initial weight. Feed consumed = feed given − feed not eaten. Δt = duration (days).
Experimental design
90-L circular fibre tanks were used in this experiment, with proper aeration (Fig. 1). Triplicate groups of fingerlings each were fed four isonitrogenous diets (above 34 % C.P) namely T-1, T-2, T-3 and T-4. Acclimatization period of 15 days was applied to all fishes in laboratory condition, with the feed of 1: 1 mixture of rice bran and mustard oil cake. Stocking rate was 25 fish/tank. Feed ingredients were cleaned under running tap-water, then sun dried for weeks and then stored in air tight plastic container in refrigerator. Diet particle size was used as 0.4 mm to 1.25 mm. Feeds were applied twice a day (08:00 a.m. and 03:00 p.m.) at the rate of 5 % wet body weight basis of the fry. Feed readjusted biweekly. Experimental trial period was of 60 days.
Sampling and data analysis
25 fishes were sampled (Randomly) every 14th day to determine fish growth rate from each treatment, by the help of glass nylon hapa. 1 h after feeding, faeces, waste particles of food and dead bodies of fish were siphoned out for measurement. The survival rate of fish was calculated at 60th day. Feed ingredients, formulated diets, fish body carcass, etc., were analysed for proximate composition following the AOAC (1995) procedures. Water quality parameters were determined by APHA (1992) procedures. All the structured designs and data were analysed using MS-Excel one-way ANOVA. This was followed by Duncan’s new multiple range test (Duncan 1955) to identify the level of significance of variance (P < 0.05) among the treatment means. Standard deviations (±SD) of treatment means were also calculated.
Results
Table 1 shows highest protein content in poultry viscera (60.67 %) that is much more than in Fishmeal (55.19 %). Highest lipid contents observed in poultry skin (12.89 %). Highest ash contents observed in poultry littre (15.98 %). In this study, the estimated protein, lipid and ash contents of poultry viscera are 60.67, 12.25 and 08.93 %, respectively those are slightly higher than the findings of Cai et al. (1994). The estimated Lipid contents of Poultry Intestine are 10.41 % which is more or less similar with the findings (07.64 %) of Tabinda and Butt (2012).
Feed formulation and proximate chemical composition of the control and experimental diet (%)
Replacement of fish meal with poultry viscera in compound diet was performed on the culture of A. testudineus for 60 days. In the feeding treatments fish meal was replaced by 33.3, 66.6, and 100 % of poultry viscera, respectively, as the experimental diets. The diet containing 100 % fish meal (0 % poultry viscera) was set as the control diet to compare performances with those fed with other experimental diets (Tables 2, 3, 4).
Notes: different levels of replacement of fish meal with poultry viscera.

Discussion
In this study, the common fish feed ingredients like fish meal, mustard oil cake, rice bran, wheat bran and wheat flour were used for proximate analysis, which are usually available throughout the year and all over India. Low cost and good quality supplementary feeds proved essential for superior practices, and it was the key demand of farmers to reduce production cost. For formulation of fish feed, information about feed ingredient’s price, nutritive value, application strategies, and its seasonal variation on quality and availability were crucial. The present study attempted to collect this information.
Due to rising cost, uncertainty and unavailability of fishmeal, it was an immense problem in modern aquaculture, especially in relation to fish nutrition, to find a desirable replacement for fish meal. Researches on poultry viscera have revealed interesting results and more works were being done by scientists from all over the world.
The analysed crude protein content of the poultry viscera was estimated in the range between 57.90 and 63.44 %, more or less similar to the findings of Fisheries Research Institute, FRI (1989). During the present study the crude lipid content was recorded in the range between 09.22 and 16.49 % in poultry viscera, more or less similar to the findings of Hasan and Amin (1997).
Triplicate groups of fingerlings each were fed four isonitrogenous (34–35 % C.P) diets namely T-1, T-2, T-3 and T-4 which is, however, similar with the treatments of Adhikary et al. (2009) and this level is higher than that of 30 % reported for A. testudineus by Chareontesprasit and Jiwyam (1996).
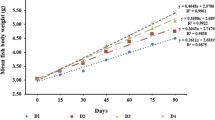
The results showed that the growth of A. testudineus fry varied significantly (P < 0.05) with different diets. At the end of feeding trial, highest S.G.R (1.39) and survival rate (85 %) were found where fish were fed on T-4 (treatment where fishmeal 100 % replaced by poultry viscera). The poorest growth rate was shown by T-1 (control treatment, where fishmeal was not replaced by poultry viscera). These results are more or less similar with the findings of Doolgindachabaporn (1994).
FCR was highest (3.82) in T-1 and gradually decreased (2.10) in T-4 with the increasing dietary protein levels. Similar trend observed in Tilapia (Jauncey 1982) and Puntius gonionotus (Wee and Ngamsnae 1987). Doolgindachabaporn (1994) found that the FCR value of A. testudineus ranges from 1.8 to 3.0. Similarly, FCR values in the present study were comparable with FCR values (01.29–01.62) in rainbow trout, by the nutritional diet of co-dried fish silage (Hardy et al. 1984). Potongkam (1972) reported that FCR of climbing perch fed on trash fish and pellet was 2.07 and 1.89, respectively.
In the present study, the water quality parameters were found to be within the acceptable level for fish culture. Recommendation for water quality usually specifies DO greater than 3 mg/l for growth of channel catfish (Weeks and Ogburn 1973). The pH values obtained in this study (7.6–7.9) fell within the suitable range according to Swingle (1967) and Boyd (2000).
My current research focused on the evaluation of poultry viscera as a prospective replacement for fish meal in the culture of Koi, A. testudineus. It is hoped that the results will reveal more clues that will justify poultry viscera as a potential replacement for fish meal. The experimental results suggested that T-4 (treatment where fishmeal 100 % replaced by poultry viscera) can be recommended for the intensive culture of A. testudineus.
Conclusion
Poultry viscera can 100 % replace fish meal potentially, in the compound diet for Koi, A. testudineus, only by proper formulation and sun-dry processing. Applying these feed fish farming becomes more profitable to the poor fish farmers by lowering the feed costs to a certain degree.
Author contribution
All authors, have made reasonable effort on all parts of the work necessary for the development of this manuscript in accordance with their expertise. All authors read and approved the final manuscript.
Abbreviations
- 90-L:
-
90 Litre capacity, of the circular fibre fish rearing tank
- cm:
-
Centimetre (Length)
- CP:
-
Crude protein
- DO:
-
Dissolved oxygen in water
- E longitude:
-
Eastern longitude
- FCR:
-
Feed conversion ratio
- FM:
-
Fishmeal
- gm:
-
Gram (weight)
- NFE:
-
Nitrogen-free extract
- N latitude:
-
Northern latitude
- PVisc:
-
Poultry viscera
- SGR:
-
Specific growth rate
- T:
-
Treatment
- Temp:
-
Temperature
- WB:
-
West Bengal
References
Adewoye SO, Ayoola PB (2010) Nutrient composition of some freshwater fishes from Ikosi dam, Ogbomoso, Nigeria. Electr J Environ Agric Food Chem 10(9):1579–4377
AOAC (Association of Official Analytical Chemicals), (1995) Official Method of Analysis, 12th edn. Association of Official Analytical Chemists, Washington 832
APHA (1992) Standard methods for the examination of water and waste-water. American Public Health Association, 1015 Eighteenth street, Washington pp 1134
Belal IEH, Al-Owaifeir A, Al-Dosari M (1995) Replacing fish meal with chicken offal silage in commercial Oreochromis niloticus (L.) feed. Aquac Res 26:855–858
Bhaskar Pratyush, Pyne Saroj Kumar, Ray Arun Kumar (2014) Evaluation of poultry viscera as potential fish feed ingredient, compared to fishmeal. Int J Curr Res 6(02):5241–5243
Boyd CE (2000) Water quality: an introduction. Kluwer, Boston
Cai T, Pancorbo OC, Barnhart HM, Sander JE, Merka WC (1994) Chemical and microbiological characteristics of poultry processing by-products, waste, and poultry carcasses during lactic acid fermentation. J Appl Poult Res 3:49–60
Cappell R, Wright S, Nimmo F (2007) Sustainable production and consumption of fish and shellfish. Environmental impact analysis. Final report of Defra LCA project. Haskoning UK Ltd, UK
Castell JD, Tiews K (1980) Report of the EIFAC, IUNS and ICES working group on the standardization of methodology in fish nutrition research. EIFAC technical paper 36. Hamburg, Federal Republic of Germany, 21–23 March, 1979, 24 pp
Chareontesprasit N, Jiwyam W (1996) Dietary protein requirement on growth for climbing perch (A. testudineus). Khon Kaen J Agri 24(3):116–120
Cho CY, Slinger SJ, Bayley HS (1982) Bioenergetics of Salmonid fishes: energy intake, expenditure and productivity. Comp Biochem Physiol B 73:25–41
Davies SJ, Williamson J, Robinson M Robert IB (1989) Practical inclusion levels of common animal by-products in complete diets for tilapia (Oreochromis mossambicus, Peters). Proc Third Int Symp on Feed Nutr Fish, Toba, Japan pp 326–332
Davis DA, Arnold CR (2000) Replacement of fishmeal in practical diets for the Pacific white shrimp, Litopenaeus vannamei. Aquaculture 185:291–298
Dong FM, Hardy RW, Haard NF, Borrows FI, Rasco BA, Fairgrieve WT, Forster IP (1993) Chemical composition and protein digestibility of poultry by-product meals for salmonid diets. Aquaculture 116:149–158
Doolgindachabaporn S (1994) Development of optimal rearing and culturing system for Climbing perch, Anabas testudineus (Bloch). Doctoral Thesis, University of Manitoba, Canada
Duncan DB (1955) Multiple range and multiple F tests. Biometrics 11:1–42
El-Sayed A-FM (1998) Total replacement of fish meal with animal protein sources in Nile tilapia, Oreochromis niloticus (L.) feeds. Aquac Res 29:275–280
Fowler LG (1991) Poultry by product meal as a dietary protein source in fall chinook salmon diets. Aquaculture 99:309–321
FRI (1989) Survey of potential fish feed ingredients of Bangladesh on the basis of their availability and biochemical composition. Research Project Report No. 1: Fisheries Research Institute, Mymensingh, Bangladesh pp. 70
Hardy RW (1996) Alternate protein sources for salmon and trout diets. Animal Feed Sci Chronol 59:71–80
Hardy RW, Shearer KD, Spinelli J (1984) The nutritional properties of co-dried fish silage in rainbow trout diets. Aquaculture 38:35–44
Hasan MR, Amin MR (1997) Effect of processing techniques on the nutritional quality of poultry offal meal. Bangladesh J Fish 20:139–144
Hastings WH (1976) Fish nutrition and fish feed manufacture. In: FAO technical conference on aquaculture, p. 13, Kyoto, Japan, FIR : Aq. Cof. 76. R 23
Henken AM, Lucas H, Tijseen PAT, Michiels MAM (1986) A comparison between methods used to determine the energy content of feed, fish and faecal samples. Aquaculture 58:195–201
Jauncey K (1982) The effect of varying dietary protein level on the growth, food conversion, protein utilization and body composition of juvenile tilapia (Tilapia mossambicus). Aquaculture 27:43–54. doi:10.1016/0044-8486(82)90108-9
Jayaram KC (1981) The freshwater fisheries of India, Pakistan, Bangladesh, Burma and Sri Lanka—a handbook. Zoological Survey of India, Calcutta
Kaushik SJ (1995) Nutrient requirements, supply and utilization in the context of carp culture. Aquaculture 129:225–241
Millamena OM (2002) Replacement of fish meal by animal by-product meals in a practical diet for grow-out culture of grouper, Epinephelus coioides. Aquaculture 204:75–84
Nengas I, Alexis MN, Davies SJ (1999) High inclusion levels of poultry meals and related by products in diets for gilthead sea bream, Sparusaurata L. Aquaculture 179:13–23
Patra BC (1993) Satiation time, appetite and daily pattern of feed intake and faces release by an air-breathing fish (A. testudineus Bloch). J Aquac Tropic 8(1):41–46
Pearson D (1976) The chemical analysis of food. Churchil Livingstone, Edinburgh, London and New York, pp 375
Pearson D (1999) Pearsons composition and analysis of foods. University of Reading, Reading
Phumee P, Hashim R, Paiko MA, Shu-Chien AC (2009) Effects of dietary protein and lipid content on growth performance and biological indices of iridescent Shark (Pangasius hypophthalmus, Sauvage 1878) fry. Aquac Res 40:456–463
Potongkam K (1972) Experiment on feeding climbing perch, Anabas testudenius (Bloch) with ground trash fish and pellets. Department of Fisheries Annual Report, Bangkok, Thailand
Saha KC (1971) Fisheries of West Bengal. West Bengal Government Press, Alipore
Steffens W (1994) Replacing fish meal with poultry by product meal in diets for rainbow trout, Oncorhynchus mykiss. Aquaculture 124:27–34
Swingle HS (1967) Standardization of chemical analysis for waters and pond muds. FAO Fish Res 4(4):397–421
Tabinda AB, Butt A (2012) Replacement of fish meal with poultry by-product meal (chicken intestine) as a protein source in grass carp fry diet. Pakistan J Zool 44(5):1373–1381
Tihn LV (2003) Culture of climbing perch in ponds with different crude protein diets, Vietnam. MS Thesis, Can Tho University
Webster CD, Thompson KR, Morgan AM, Grisby EJ, Gannam AL (2000) Use of hempseed meal, poultry by-product meal and canola meal in practical diets without fish meal for sunshine bass (Moronechrysop × M. saxatilis). Aquaculture 188:299–309
Wee KL, Ngamsnae P (1987) Dietary protein requirement of fingerlings of the herbivorous Puntius gonionotus (Bleeker). Aquac Fish Manag 18(2):121–129
Weeks JP, Ogburn CB (1973) Catfish production guide. Circ. E-18, Alabama Cooperative Extension Service, Auburn University, Auburn, USA
Acknowledgments
The authors are thankful to the Head, Department of ASEPAN, Palli Siksha Bhavana, Visva-Bharati, Sriniketan and Department of Zoology, Siksha Bhavana, Visva-Bharati Santiniketan, for providing necessary facilities for this research.
Conflict of interest
The authors declare that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Bhaskar, P., Pyne, S.K. & Ray, A.K. Growth performance study of Koi fish, Anabas testudineus (Bloch) by utilization of poultry viscera, as a potential fish feed ingredient, replacing fishmeal. Int J Recycl Org Waste Agricult 4, 31–37 (2015). https://doi.org/10.1007/s40093-014-0082-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40093-014-0082-y