Abstract
The method of micro bubbles is widely applied in the fields of water and soil treatment. A novel treatment method of NO in flue gas through a gas–liquid two-phase system formed by micro bubbles is proposed in this study. The system depends on the generation of hydroxyl radicals. The NO removal performance of the micro gas–liquid dispersion system induced by catalysts and O3 was explored and the reaction pathways were elucidated. Micro bubbles, Fe2+, and Mn2+ in solution improved NO removal performance significantly. Salinity and surfactants affected the removal performance of NO by altering micro bubbles. In the presence of Fe2+, the NO removal rate reached 65.2% at pH 5, 75.8% under 0.5 g/L NaCl and 82.1% under 6 mg/L sodium dodecyl sulfate. In the presence of Mn2+, the NO removal rate reached 69.2% at pH 5, 83.2% under 0.5 g/L NaCl and 92.3% under 6 mg/L sodium dodecyl sulfate. However, in the presence of both Mn2+ and Fe2+, NO conversion rate was 93.2%. The NO removal rate in the presence of O3 was further improved under the same conditions. The study provides the basis for the application and development of micro bubbles in flue gas treatments for NO removal. The results can help to solve the problems of high operating cost, large oxidant consumption, secondary pollution, and high energy consumption in traditional NO removal methods.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Micro bubbles (MBs) refer to a kind of bubble mixture whose bubble diameter is between several hundred nanometers and tens of micrometers [1,2,3]. MBs’ residence time in water is tens of seconds to several days [4, 5]. Negatively charged ions (such as OH−) in water are prone to be adsorbed on the surface of MBs, so MBs carry some negative charges [6, 7]. As the bubble diameter reaches micrometers or even nanometers, the surface tension of the gas–liquid interface compresses the bubbles, thus increasing the specific surface area of bubbles and the oxygen transfer efficiency between gas and liquid [8]. The diameter of MBs is small and the bubble pressure is high. When MBs rupture, they release high energy and hydroxyl radicals [9]. Surfactants, salinity, and pH affect the properties of MBs [10]. Surfactants make them more stable [11]. Under the conditions of low salinity and low pH, the stability of the MBs is enhanced. Till now, MBs have been extensively explored in environmental applications, especially in surface water restoration [12, 13], agricultural production [14], and ozone oxidation [15]. The NO removal by the micro bubbles gas–liquid dispersion system (MBGLS) has not been reported yet.
NOx is one of the major air pollutants and the NO concentration in flue gas is sometimes as high as 90% [16]. Due to insoluble NO [17], it is necessary to oxidize NO into soluble NO2 in wet denitration processes. NO oxidation methods mainly include photocatalysis [18], plasma oxidation [19], strong-oxidant oxidization [20], and selective catalytic oxidation (SCO) [21, 22]. SCO utilizing catalysts and O2 in flue gas can be combined with traditional wet absorption processes to achieve efficient and integrated desulfurization and denitration and has become the most promising NO oxidation method in industrial applications [23]. However, in order to realize high denitration rate, the NO oxidation methods should be improved in the following three aspects: First, it is necessary to improve the resistances of catalysts to steam, sulfur, and other pollutants so as to ensure the stability of NO oxidation rate. Second, the catalyst cost should be lowered. Third, it is necessary to increase the recovery rate of catalysts. Advanced oxidization processes can be divided into two categories: gas phase oxidation processes and the liquid phase oxidation processes. NO oxidation reactions happen in the gas phase with common oxidants, such as O2, O3, Cl2, and CIO2−, or in the liquid phase with oxidants [24] such as Na2S2O8 [25], KMnO4 [26], NaClO2 [27], and H2O2 [28]. The absorption probability of NOx can be increased by the addition of various agents [29, 30]. NOx removal by the oxidation–absorption method has been extensively explored. However, several problems in the NO oxidation–absorption methods remain to be resolved, such as high operation cost, non-recyclable absorption liquid, large oxidant consumption, and the comprehensive use of absorption liquid. Hence, it is imperative to develop a denitration method with high denitration efficiency, low energy consumption, non-secondary pollution, less reagent consumption, high utilization rate of oxidant and low investment.
In order to achieve the high denitration rate, NO oxidation–absorption removal processes should be improved in the following aspects. First, contact time between NO and oxidant should be long. Second, the oxidation ability of oxidants should be strong. Third, the oxidation reactions or catalysts should not be affected by various pollutants. Fourth, the denitration process should prevent regenerated nitric oxide from escaping for the application in different flue gas environments. The properties of MBs meet the above four conditions. In the study, a new MBGLS generation process utilizing MB generator is proposed to treat NO through inhaling water and the mixed gases of NO and O3/air. In the process, MBGLS is sprayed into the oxidation–absorption tower, in which NO is oxidized and absorbed. The effects of reaction parameters such as the amount of intake (O3, NO), transition metal ion catalyst (Mn2+ and Fe2+), concentration of salt medium (NaCl), and surfactant (sodium dodecyl sulfate, SDS) on the rate of NO oxidation–absorption were explored. This study provides a laboratory theoretical basis for the industrialization of flue gas treatment by micro-nano bubble technology and is expected to achieve flue gas reduction and resource utilization.
Materials and methods
Experimental materials
The main experimental devices include micro bubble generator (XZCP-K-0.75), ozone generator (CFT-5G), glass rotor flow meter, UV–Vis spectrophotometer (N4, Shanghai INESA Scientific Instrument Co.), steady-state/transient fluorescence spectrometer (QM/TM*, the United States), and portable pH meter (MODEL 6010). Sodium hydroxide (NaOH), hydrochloric acid (HCl), sodium chloride (NaCl), SDS, ferrous sulfate (FeSO4·7H2O), manganese sulfate (MnSO4·4H2O), NO mixture (N2), and high-purity O2 were used in the experiments. All chemicals were purchased from Sinopharm Chemical Reagent Co. and used without further purification.
Experimental devices
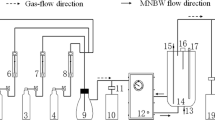
Figure 1 is a schematic diagram of an apparatus for NO oxidation and absorption by MBGLS. The entire device consists of a gas distribution system, a water inlet system, a micro bubble generator, and an oxidation–absorption tower. Through controlling the intake of different gases by the gas flow meter (Fig. 1d), NO and O3/air are mixed in a gas mixer (Fig. 1f) and then passed into a micro bubble generator (Fig. 1g) together with water (Fig. 1i) to form MBGLS in the oxidation absorber (Fig. 1j). The tail gas is further cleaned by the exhaust gas absorption unit before entering the fume hood.
In the oxidation–absorption process, air/O3 was used as the gas-phase medium and the liquid-phase medium was prepared by adding Mn2+, Fe2+, SDS or NaCl solution into water. The oxidation absorption tower is a customized cylindrical absorption column with a volume of 40 L. The inlet flow rate of micro bubbles generator is 12 L/min. The air intake volume is 2 L/min and the water inlet pressure is 0.2 MPa. After 3-min stable operation, the concentrations of NO3− and NO2− were determined to calculate NO removal rates.
Methods
Air/O3 and NO were mixed according to different proportions and then added into the micro bubble generator together with the absorption liquid to prepare MBGLS, which was sprayed into the oxidation–absorption tower, where MBs gradually collapsed and disappeared so that the NO absorption process was completed. In the process, nitrate nitrogen, nitrite nitrogen, and hydroxyl radical in the solution in the oxidation absorption tower were measured by the steady-state/transient fluorescence spectrometer and the UV absorption spectrophotometry to calculate denitration rates.
The effects of NO intake (1250 ppm, 2500 ppm, 3750 ppm, 5000 ppm, and 6250 ppm), O3 intake (1675 ppm, 2500 ppm, 5000 ppm, 10,000 ppm, and 15,000 ppm), pH (3, 5, 7, 9 and 11), NaCl concentrations (0.1 g/L, 0.3 g/L, 0.5 g/L, 0.7 g/L, and 0.9 g/L), SDS concentrations (2 mg/L, 4 mg/L, 6 mg/L, and 8 mg/L), and transition metal ion concentrations (0.5 mmol/L, 1 mmol/L, 2 mmol/L, 3 mmol/L, and 4 mmol/L) on NO oxidation–absorption efficiency in the MBGLS were explored. Afterwards, the mechanism of NO oxidation–absorption by MBs oxidization process was elaborated.
·OH test
First, an excess of terephthalic acid (TA) was added into a colorimetric tube and directly passed through the newly generated 25 mL of MBs. The ·OH immediately reacted with TA to form 2-hydroxyterephthalic acid when the micron bubble broke. After 3 min, the bubbles in the colorimetric tube disappeared and the test was performed with the steady-state/transient fluorescence spectrometer.

The capture agent TA is not a fluorescent agent and TAOH has a strong absorption peak at an excitation spectrum of 325 nm and an emission wavelength of 415 nm [31]. TA captures hydroxyl radicals to form TAOH. The content of hydroxyl radicals was determined by a fluorescence spectrometer.
NO removal rate calculation method
MBs gradually collapsed and disappeared after a certain period, so that the NO removal process was completed in the oxidation–absorption tower (Fig. 1j). In this study, the concentrations of NO2− and NO3− in the absorption solution were determined by the UV absorption spectrophotometer.
In the experiment, NO2− was not detected in the absorption solution, so the NO removal rate was calculated with the increase in the quantity of NO3− in the absorption system. The NO removal rate is calculated as follows:
where h is the NO removal rate, %; c1 is the mass concentration of NO3− in N in the absorption liquid, mg/L; q1 is the inflow, L/min; t is the system running time, min; m1 is the mass of intake NO (in N); M is the molar mass of N (g/mol) and the molar volume of gas under standard conditions is 22.4 L/mol; A is NO gas volume fraction, ppm; T is room temperature, °C; P1 is the pressure after gas depressurization, Pa; P0 is the atmospheric pressure, Pa; q2 is the gas flow rate, mL/min; t is the system running time, min.
Statistical analysis
Experimental data were analyzed in Statistical Product and Service Solutions 19.0. One-way analysis of variance with a 95% confidence interval and Dennett’s post-test was used to determine whether the significant differences between the mean of the pristine group and the mean of each experimental group (n = 3, P < 0.05).
Results and discussion
Proposed reaction pathways
In the widely concerned advanced oxidation processes (AOPs) for the removal of NOx from flue gas, the transition-metal and ·OH serve as effective oxidants [32]. Fe2+ and Mn2+ ions activate MBs, which can generate ·OH as the main oxidant for the conversion of NO [17]. Hence, NO removal in aqueous solutions is also thought to be dependent on the consumption of dissolved NO by various reactive species generated after MBs’ breakage, thus maintaining the driving force required for the absorption process. The detailed proposed pathways for the MBGLS are illustrated in Fig. 2.
As shown in Fig. 2, the chemistry of NO consumption by MBGLS is complex, since it takes place through direct electron transfer in the free radical oxidation reactions via ·OH, and the direct reaction of the NOx species with O3, Mn2+ and Fe2+. The main reactions involved in the oxidation and absorption of NO are provided as follows:
Mechanism of NO by oxidative absorption of MBs
Figure 3 shows the result of ·OH measured by the steady-state/transient fluorescence spectrometer. The content of hydroxyl radicals in MBGLS is much higher than that in water, indicating that MBs activate dissolved oxygen in water, generate hydroxyl radicals, and finally increase the NO oxidation efficiency. This, residence time of MBs in the liquid phase is prolonged and the mass transfer between gas and liquid is enhanced.
Effects of transition metal ions on NO removal rate
To determine the effects of transition metal ionic concentration on NO removal rate, we prepared influent solutions with different Mn2+ (or Fe2+) concentrations (0.5 mmol/L, 1 mmol/L, 2 mmol/L, 3 mmol/L, and 4 mmol/L) and different Mn2+/Fe2+ ratios (0.5, 1, 2, 3 and 4). Then, we determined the concentrations of NO3− and NO2− in the oxidation–absorption tower to calculate NO removal rates. After the addition of Fe2+ and Mn2+, when the ion concentration in MBGLS was increased below 2 mmol/L, the removal rate was significantly improved (Fig. 4). Free radicals oxidized Fe [II] and Mn [II] into Fe [III], Mn [III], and oxygen radicals after the addition of Fe2+ and Mn2+ in the liquid phase. Therefore, Fe [III] and Mn [III] oxidized NO into NO2 as a catalyst to accelerate the removal of NO. When the ion concentration was 2 mmol/L, the NO removal rate reached 63.4%. When the ion concentration further increased above 2 mmol/L, the NO removal rate decreased. Both Fe2+ and Mn2+ at higher concentrations were hydrolyzed, and fine particles produced by hydrolysis were found adhered to the gas surface. It was difficult for flue gas inside bubbles to interact with hydroxyl radicals in the liquid phase, thus resulting in a decrease in the efficiency. Transition metal ions could react with O3 molecules to produce ·O3−, thus leading to a chain reaction producing ·OH, as indicated in Eqs. (4) and (5).
Under the same conditions, after the addition of metal ions, the removal rate of NO obtained after adding Mn2+ was higher than that obtained after the addition of Fe2+ (Fig. 4). The difference may be interpreted as follows: The catalytic ability of Mn2+ is stronger than that of Fe2+. According to Eqs. (11)–(16), the potential of Mn3+/Mn2+ is 1.5 V higher than that of Fe3+/Fe2+ (0.77 V) [33]. Moreover, Fe2+ is easier to be hydrolyzed than Mn2+ and the mass transfer efficiency is reduced.
When the metal ion concentration was low, O3 molecules and metal ions were combined to produce hydroxyl radicals through chain reactions. The hydroxyl radicals oxidized NO into nitrogen oxides, which were then easily absorbed. Therefore, NO removal rate was increased. When the concentration of metal ions was greater than 2 mmol/L, the O3 molecules decomposed faster and generated more oxygen. At this point, the dissolved oxygen solution was saturated and the NO removal rate does not increase.
When Mn2+/Fe2+ concentration ratio was 1, the NO removal rate was up to 93.2%, which was 30.1% higher than that obtained after the addition of Mn2+ alone, and 33.2% higher than that obtained after the addition of Fe2+ alone. This proved that Mn2+ and Fe2+ showed the synergistic effect on the catalytic NO oxidation removal. In the experiment, when more Fe2+ was added, the hydrolysis degree of Fe2+ was aggravated. At this stage, the solution turned into a red-brown turbid liquid, thus hindering the contact of NO with ozone and decreasing NO removal rate.
Effects of pH on NO removal rate
To determine the effects of pH on NO removal rate, we prepared influent solutions with different pH values (3, 5, 7, 9 and 11) and determined the concentrations of NO3− and NO2− in the oxidation–absorption tower to calculate NO removal rates. When pH increased below 5, NO removal rate increased (Fig. 5). When the pH further increased above 5, NO removal rate decreased. With the addition of Fe2+ or Mn2+ ions, the NO removal rate increased. When the pH was low, due to the high acidity of the liquid phase, OH− accumulated on the surface of the MBs was consumed, thus resulting in the decreased surface charge on the MBs. Bubbles ruptured easily and gas–liquid contact time was decreased, thus eventually resulting in the decreased NO removal rate. Therefore, when the pH was close to 5, dissolved oxygen in water was also relatively high, thus promoting the oxidation of NO into NO2. Fe2+ and Mn2+ were greatly hydrolyzed in water, thus resulting in turbidity in the liquid phase and affecting the gas–liquid mass transfer. Ion hydrolysis decreased the ion concentration and eventually affected the ion catalysis efficiency. Under the condition of higher pH, the oxidation potential of reactive oxygen species became lower and the negative charges carried on the surface of the bubbles were easily repelled from the OH− ions in the solution, thus increasing bubble movements in water as well as the probability of bubble collision. Therefore, it eventually led to the rupture of bubbles, affected bubble residence time, and decreased NO oxidization and absorption.
The stability of ozone in water was strongly influenced by pH in O3 MBGLS. At lower pH, ozone decomposed more slowly in water. Ozone molecules were oxidized and NO removal rate also increased. When the pH value was lower than 5, since the oxidation–reduction potential of O3 was lower than that of ·OH, NO removal rate was not high. As the solution pH increased, O3 reacted with OH− to form hydroxyl radicals and the NO removal rate increased. When the solution was alkaline, Fe2+ and Mn2+ were greatly hydrolyzed in water and the oxidization ability of reactive oxygen was decreased, thus decreasing the NO removal rate.
Effects of NaCl and pH concentration on NO removal rate
To determine the effects of NaCl concentration on NO removal rate, we prepared influent solutions with different NaCl concentrations (0.1 g/L, 0.3 g/L, 0.5 g/L, 0.7 g/L and 0.9 g/L) and determined the concentrations of NO3− and NO2− in the oxidation–absorption tower to calculate NO removal rates. As shown in Fig. 6a, with the increase in NaCl concentration, NO removal rate gradually increases. When the NaCl concentration was 0.5 g/L, NO removal rates under three conditions (Mn2+, Fe2+, and control without catalyst) were, respectively, 75.8%, 83.2%, and 47.4%. As the salinity of the solution increased, dissolved oxygen increased and peaked under a NaCl concentration of 500 MBG/L (Fig. 6b). The result was consistent with experimental results. When the NaCl concentration further increased, the NO absorptivity decreased. As the hydration energy of Na+ (− 406 kJ/mol) is close to that of CI− (− 317 kJ/mol), under the low NaCl concentration (0.1–0.5 g/L), OH− ions on bubble surface are combined with Na+ ions. Therefore, the zeta potential of bubbles decreases and the gas–liquid oxygenation rate is increased. In this way, NO removal rate increases. An appropriate salinity increases liquid viscosity and bubble surface tension, enhances bubble stability, and extends residence time in water. With the increase in NaCl concentration, Cl− is gradually accumulated on bubble surface, thus increasing gas dissolution resistance and decreasing NO removal rate.
To determine the effects of SDS concentration on NO removal, we prepared influent solutions with different SDS concentrations (2 mg/L, 4 mg/L, 6 mg/L and 8 mg/L) and determined the concentrations of NO3− and NO2− in the oxidation–absorption tower to calculate NO removal rates. NO removal rate increased with the increase in SDS concentration (Fig. 6). At the SDS concentration of 6 mg/L, when Mn2+ and Fe2+ were, respectively, added to the system, the NO removal rates reached 82.1% and 92.3%, respectively. However, when the SDS concentration was further increased, the NO removal rate decreased. After the addition of the surfactant (SDS) in water, the resulting MB size was significantly reduced. With the increase in SDS concentration, the bubble size decreased and the internal pressure of bubbles increased. The energy released at the moment of bubble rupturing produced a large number of free radicals, which accelerated oxidation and absorption of NO. When the SDS concentration was too high, the bubble size was too small and the stability was weakened. Residence time in water phase was too short and not conducive to the gas–liquid contact, thus resulting in the slightly decreased NO removal rate.
Smaller bubbles indicate the larger specific surface area and the larger gas–liquid contact area. O3 generated by ozone decomposition is beneficial to the gas–liquid exchange, as it can accelerate the chain reaction rate of ·OH, generate more free radicals, and promote the oxidative absorption of NO. In addition, smaller bubbles indicate the greater pressure inside bubbles and the more energy released when rupturing. Therefore, it increases molecular movement and NO removal rate.
Effects of NO concentration on NO removal rate
To determine the effects of inlet flow rate on NO removal rate, we prepared the inlet gas with different NO concentrations (1250 ppm, 2500 ppm, 3750 ppm, 5000 ppm and 6250 ppm). Then, we determined the concentrations of NO3− and NO2− in the oxidation–absorption tower to calculate NO removal rates. Figure 7a shows the effects of NO concentration on NO removal rate. The NO removal rate decreased as the NO intake flow rate increased. However, after Fe2+ and Mn2+ ions were ,respectively, added, the removal rate of NO at a concentration of 1250 ppm reached 100%, which was 50% higher than that obtained without the addition of Fe2+ and Mn2+ ions. Compared with NO removal rate in the conventional bubble column, the NO removal rate in MBGLS was significantly increased. NO is extremely difficult to be dissolved in water. According to the double-film theory, the dissolution process of NO in water is controlled by a liquid film. NO2 as the oxidation product of NO, is soluble in water and its dissolution process is controlled by the gas film. With the increase in NO concentration, the mass transfer efficiency between NO and liquid phase did not change greatly [34]. Therefore, the NO removal rate decreased with the increase in NO concentration. However, after transition metal ions such as Fe2+ and Mn2+ were, respectively, added into the absorption liquid, they made the reaction faster as they played a catalytic role in the reaction by generating the free radical chain (Eqs. (11)–(16)). Therefore, hydroxyl radicals oxidized NO at a much faster rate. The removal rate of NO increased significantly after the addition of transition metal ions.
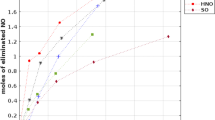
When [O3]/[NO] was 0.5, NO removal rate reached 52.6% (Fig. 7b). With the increase in [O3]/[NO] (more than 0.5), the NO removal rate increased slightly. In the gas phase, NO was oxidized by O3 into high-valence NOx, such as NO2. The high-valence NOx became soluble in water to form nitric acid. As indicated in Eq. (9), the most suitable mole ratio of [O3]/[NO] for the oxidation reaction between NO and O3 is 1. However, according to Eqs. (4)–(9), oxygen atom produced by ozone decomposition activates water into hydroxyl radicals. Therefore, hydroxyl radicals reacted with NO to generate NO2 and reduced the ozone consumption. Bubbles ruptured and generated free radicals, thus enhancing NO oxidation and absorption. According to Eqs. (4)–(10), with the increase in [O3]/[NO], a large amount of nitric acid accumulates in the solution, thus resulting in a drop in the pH of the solution. When [O3]/[NO] was larger than 1, the dissolution of NO2 was limited, but the removal rate of NO was not greatly affected.
MBs were generated with air/O3 and water. The denitration efficiency of the two MBGLS was compared under the same conditions. The parameters of the air MBGLS are provided as follows: 5000 ppm NO, pH 5, 0.5 g/L NaCl, 6 mg/L SDS and [Mn2+/Fe2+] = 1 (concentrations of both metal ions were 2 mmol/L). For O3 MBGLS, the ratio of O3 and NO was 0.5 and the other conditions were the same. Under these conditions, NO oxidization and absorption performances of O3 and air MBGLS were compared (Fig. 7b).
As shown in Fig. 7b, under the optimal conditions, the NO removal rate in O3 MBGLS is significantly higher than that of the air MBGLS. The difference may be interpreted as follows: First, in O3 MBGLS, the ozone oxidation process of NO can be divided into two steps. In the first step, NO is oxidized by ozone to NO2 in the interior of the MBs (gas phase). In the second step, free radicals are generated by ozone decomposition in water, partial NO in the liquid phase is oxidized into NO2 and eventually absorbed by the liquid. Similarly, in the air MBGLS, there are two oxidation processes. In the first step, oxygen has lower oxidation performance than ozone. In the second step, compared with ozone MBGLS, air MBGLS do not generate extra free radicals (except free radicals generated when bubbles burst). In summary, under the same conditions, the removal of nitrogen oxides by the ozone MBGLS proved to be much better than that of the air MBGLS.
Conclusions
This study demonstrates the feasibility of the efficient removal of NO from flue gas with the air/O3 MBGLS. The effects of O3 concentration, transition metal ion concentration, pH, NaCl concentration, SDS concentration, Mn2+ to Fe2+ ratio, and other factors on NO removal rate were explored. MNBLS can effectively remove NO, realize the synergistic desulfurization and denitrification and reduce various pollution gases such as VOCs in the future. The conclusions are stated below.
In the air and NO MBGLS (1250 ppm NO and pH 7), the NO removal rate reached 50.1%. Under 3750 ppm NO, after Fe2+ and Mn2+ ions were, respectively, added, the removal rates, respectively, reached 60.0% and 63.1%. Under the conditions of 3750 ppm NO, pH 7, 0.5 g/L NaCl, and 6 mg/L SDS, when 2 mmol/L Fe2+ and 2 mmol/L Mn2+ ions were, respectively, added, the NO removal rates reached 82.1% and 92.3%, respectively. When the metal ion ratio [Mn2+/Fe2+] was 1, the highest NO removal rate of the system was 93.2%.
In O3 and NO MBGLS, NO oxidation was better than that in air and NO MBGLS. When [O3]/[NO] was 0.5, the NO removal rate reached 52.6%. When 2 mmol/L Fe2+ and 2 mmol/L Mn2+ were, respectively, added into the O3 and NO MBGLS, the NO removal rates reached 61.3% and 63.4%, respectively. At pH 5, when Fe2+ and Mn2+ were, respectively, added, the NO removal rates were calculated to be 65.2% and 69.2%, respectively. Under 0.5 g/L NaCl, when Fe2+ and Mn2+ were, respectively, added, NO removal rates reached 69.4% and 72.1%, respectively. Under 6 mg/L SDS, when Fe2+ and Mn2+ were, respectively, added, NO removal rates reached 72.3% and 74.9%, respectively. O3 MBGLS was better than air MBGLS. However, in both systems, the influences of various factors on NO removal efficiency was basically the same.
Mn2+ and Fe2+ exhibited a synergistic effect on the catalytic NO oxidation and removal. After the addition of Fe2+ and Mn2+ in the liquid phase, free radicals were generated due to MBs rupturing and converted Fe [II] and Mn [II] into Fe [III] and Mn [III]. Therefore, Mn2+ and Fe2+ acted as a catalyst to accelerate NO removal. Appropriate NaCl concentration increased the liquid viscosity, bubble surface tension, bubble stability, and residence time in water. The addition of SDS decreased the bubble size and increased the internal pressure. When the MBs burst, the instantaneous energy released generated a large amount of free radicals, which accelerated oxidation and removal of NO.
References
Attard P, Moody MP, Tyrrell JWG (2002) Nanobubbles: the big picture. Phys A 314(1):696–705. https://doi.org/10.1016/S0378-4371(02)01191-3
Pérez Garibay R, Martínez Ramos E, Rubio J (2012) Gas dispersion measurements in microbubble flotation systems. Miner Eng 26(1):34–40. https://doi.org/10.1016/j.mineng.2011.10.006
Temesgen T, Bui TT, Han M, Kim TI, Park H (2017) Micro and nanobubble technologies as a new horizon for water-treatment techniques: a review. Adv Colloid Interface Sci 246:40–51. https://doi.org/10.1016/j.cis.2017.06.011
Ushikubo FY, Furukawa T, Nakagawa R, Enari M, Makino Y, Kawagoe Y, Shiina T, Oshita S (2010) Evidence of the existence and the stability of nano-bubbles in water. Colloids Surf A 361(1):31–37. https://doi.org/10.1016/j.colsurfa.2010.03.005
Azevedo A, Etchepare R, Calgaroto S, Rubio J (2016) Aqueous dispersions of nanobubbles: generation, properties and features. Miner Eng 94:29–37. https://doi.org/10.1016/j.mineng.2016.05.001
Yang C, Dabros T, Li D, Czarnecki J, Masliyah JH (2001) Measurement of the zeta potential of gas bubbles in aqueous solutions by microelectrophoresis method. J Colloid Interface Sci 243(1):128–135. https://doi.org/10.1006/jcis.2001.7842
Takahashi M (2005) ζ potential of microbubbles in aqueous solutions: electrical properties of the gas–water interface. J Phys Chem B 109(46):21858–21864. https://doi.org/10.1021/jp0445270
Li H, Hu L, Song D, Lin F (2014) Characteristics of micro-nano bubbles and potential application in groundwater bioremediation. Water Environ Res 86(9):844–851
Tada K, Maeda M, Nishiuchi Y, Nagahara J, Hata T, Zhuowei Z, Yoshida Y, Watanabe S, Ohmori M (2014) ESR measurement of hydroxyl radicals in micro-nanobubble water. Chem Lett 43(12):1907–1908. https://doi.org/10.1246/cl.140691
Yang W, Zhang R, Chen B, Duprez D, Royer S (2012) New aspects on the mechanism of C3H6 selective catalytic reduction of NO in the presence of O2 over LaFe1–x(Cu, Pd)xO3–δ perovskites. Environ Sci Technol 46(20):11280–11288. https://doi.org/10.1021/es302240m
Huaâ W, Ku Y, Shiâ Y, Chingâ Y (2007) Effect of sodium dodecyl sulfate (SDS) on bubble characteristics and ozone transfer in a bubble column. J Chin Inst Chem Eng 30(1):155–161. https://doi.org/10.1080/02533839.2007.9671239
Agarwal A, Ng WJ, Liu Y (2011) Principle and applications of microbubble and nanobubble technology for water treatment. Chemosphere 84(9):1175–1180. https://doi.org/10.1016/j.chemosphere.2011.05.054
Hu L, Xia Z (2018) Application of ozone micro-nano-bubbles to groundwater remediation. J Hazard Mater 342(15):446–453. https://doi.org/10.1016/j.jhazmat.2017.08.030
Honghui S, Xiyun J, Shufang W, Weihua G, Khaled SM, Kaihua L (2018) Effects of micro-nano bubble aerated irrigation and nitrogen fertilizer level on tillering, nitrogen uptake and utilization of early rice. Plant Soil Environ 64(7):297–302. https://doi.org/10.17221/240/2018-PSE
Jabesa A, Ghosh P (2017) Removal of dimethyl phthalate from water by ozone microbubbles. Environ Technol 38(16):2093–2103. https://doi.org/10.1080/09593330.2016.1246610
Wu Q, Sun C, Wang H, Wang T, Wang Y, Wu Z (2018) The role and mechanism of triethanolamine in simultaneous absorption of NOx and SO2 by magnesia slurry combined with ozone gas-phase oxidation. Chem Eng J 341(1):157–163. https://doi.org/10.1016/j.cej.2018.01.150
Adewuyi YG, Sakyi NY (2013) Simultaneous absorption and oxidation of nitric oxide and sulfur dioxide by aqueous solutions of sodium persulfate activated by temperature. Ind Eng Chem Res 52(33):11702–11711. https://doi.org/10.1021/ie401649s
Zhou Y, Li W, Zhang Q, Yan S, Cao Y, Dong F, Wang F (2017) Non-noble metal plasmonic photocatalysis in semimetal bismuth films for photocatalytic NO oxidation. Phys Chem Chem Phys 19(37):25610–25616. https://doi.org/10.1039/C7CP04359G
Jõgi I, Levoll E, Raud J (2016) Effect of catalyst placement on the plasma-catalytic oxidation of NO. Catal Lett 147(2):566–571. https://doi.org/10.1080/02533839.2007.9671239
Li S, Li K, Hao J, Ning P, Tang L, Wang C (2017) Simultaneous adsorption/oxidation of NO and SO2 over Al-Cu composite metal oxides supported on MCM-41 at low temperature. J Chem Eng Jpn 50(5):376–382. https://doi.org/10.1252/jcej.15we131
Zhao Y, Wang H, Wang T (2016) Adsorption of NO from flue gas by molecularly imprinted adsorbents. Chem Eng J 306:832–839. https://doi.org/10.1016/j.cej.2016.08.023
Li H, Guo SJ, Shin K, Wong MS, Henkelman G (2019) Design of a Pd–Au nitrite reduction catalyst by identifying and optimizing active ensembles. ACS Catal 9(9):7957–7966. https://doi.org/10.1021/acscatal.9b02182
Zeng Y, Wang T, Zhang S, Wang Y, Zhong Q (2017) Sol–gel synthesis of CuO–TiO2 catalyst with high dispersion CuO species for selective catalytic oxidation of NO. Appl Surf Sci 411:227–234. https://doi.org/10.1016/j.apsusc.2017.03.107
Guo RT, Hao JK, Pan WG, Yu YL (2015) Liquid phase oxidation and absorption of NO from flue gas: a review. Sep Sci Technol 50(2):310–321. https://doi.org/10.1080/01496395.2014.956761
Khan NE, Adewuyi YG (2010) Absorption and oxidation of nitric oxide (NO) by aqueous solutions of sodium persulfate in a bubble column reactor. Ind Eng Chem Res 49(18):8749–8760. https://doi.org/10.1021/ie100607u
Fang P, Cen C, Wang X, Tang Z, Tang Z, Chen D (2013) Simultaneous removal of SO2, NO and Hg0 by wet scrubbing using urea + KMnO4 solution. Fuel Process Technol 106:645–653. https://doi.org/10.1016/j.fuproc.2012.09.060
Mondal MK, Chelluboyana VR (2013) New experimental results of combined SO2 and NO removal from simulated gas stream by NaClO as low-cost absorbent. Chem Eng J 217:48–53. https://doi.org/10.1016/j.cej.2012.12.002
Collins MM, Cooper CD, Dietz JD, Clausen CA III, Tazi LM (2001) Pilot-scale evaluation of H2O2 injection to control NOx emissions. J Environ Eng 127(4):329–336. https://doi.org/10.1061/(ASCE)0733-9372(2001)127:4(329)
Zhang WX, Jiang F (2019) Membrane fouling in aerobic granular sludge (AGS)-membrane bioreactor (MBR): effect of AGS size. Water Res 157:445–453. https://doi.org/10.1016/j.watres.2018.07.069
Chen WS, Mo JH, Du X, Zhang ZE, Zhang WX (2019) Biomimetic dynamic membrane for aquatic dye removal. Water Res 151:243–251. https://doi.org/10.1016/j.watres.2018.11.078
Charbouillot T, Brigante M, Mailhot G, Maddigapu PR, Minero C, Vione D (2011) Performance and selectivity of the terephthalic acid probe for OH as a function of temperature, pH and composition of atmospherically relevant aqueous media. J Photochem Photobiol A 222(1):70–76. https://doi.org/10.1016/j.jphotochem.2011.05.003
Liu Y, Adewuyi YG (2016) A review on removal of elemental mercury from flue gas using advanced oxidation process: chemistry and process. Chem Eng Res Des 112:199–250. https://doi.org/10.1016/j.cherd.2016.06.024
Sottmann J, Nataf L, Chaix L, Pralong V, Martin C (2018) Playing with the redox potentials in ludwigite oxyborates: Fe3BO5 and Cu2MBO5 (M = Fe, Mn, and Cr). J Phys Chem C 122(30):17042–17048. https://doi.org/10.1021/acs.jpcc.8b03734
Han Z, Yang S, Pan X, Zhao D, Yu J, Zhou Y, Xia P, Zheng D, Song Y, Yan Z (2017) New experimental results of NO removal from simulated flue gas by wet scrubbing using NaClO solution. Energy Fuels 31(3):3047–3054. https://doi.org/10.1021/acs.energyfuels.6b03062
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant number U1660107).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sun, H., Yang, G., Aftab, T.B. et al. Direct catalytic oxidation and removal of NO in flue gas by the micro bubbles gas–liquid dispersion system. Int J Ind Chem 11, 11–21 (2020). https://doi.org/10.1007/s40090-019-00198-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40090-019-00198-6