Abstract
The inhibitive action of plant leaf extracts, Crataegus oxyacantha (Hawthorn) and Prunus Avium (Sweet Cherry) on the corrosion of mild steel in 0.5 M HCl solution was investigated using open circuit potential-time measurements (OCP), potentiodynamic polarization and electrochemical impedance spectroscopy (EIS) techniques. Functional groups of these plants’ leaf extracts and their absorption bands were identified by Fourier transform infrared spectroscopy (FTIR) and Ultra-Violet Spectrophotometer (UV), respectively. The leaf extracts showed good inhibition efficiency in hydrochloric acid solution. Potentiodynamic polarization curves revealed that Crataegus oxyacantha and Purnus Avium plants leaves extracts acted as mixed type inhibitors. Theoretical fitting of different isotherms, Langmuir, Florry–Huggins and the kinetic–thermodynamic models was tested to describe the mode of inhibitors’ adsorption on mild steel surface. UV spectra proved that the inhibiting action takes place through simple physical adsorption of the extracts molecules on mild steel surface.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mild steel has been extensively used in several applications including constructions of tanks, petroleum refineries equipment and flow lines as well as transmission pipelines due to its ease of fabrication and low cost [1,2,3,4,5,6]. However, it is vastly exposed to corrosion and deterioration especially in acidic media. In metallurgical industry, hydrochloric acid is widely applied in various processes such as pickling and descaling of metals, chemical or electrochemical processes in oil refinery as well as deactivation of equipment in atomic power establishments’. Various corrosion controlling methods were used to protect metals such as protective coatings, cathodic protection and the use of corrosion inhibitors [7]. Among these methods, the latter is one of the most practical methods especially in acidic media. Such inhibitors can significantly decrease the corrosion rate when added to a corrosive environment in small concentrations [8,9,10].
Most of the organic compounds containing nitrogen, oxygen and phosphorus atoms are expected to act as effective corrosion inhibitors of different metals and alloys [11]. Unfortunately, nowadays the use of such compounds is restricted due to their high cost and toxicity for both human and environment. Recently, efforts are directed towards the use of plant extracts as corrosion inhibitors. Such extracts consist of diverse natural ingredients that are eco-friendly, easily available and of low cost [2].
Numerous researchers reported the successful use of natural plant extracts on the corrosion inhibition of mild steel in different media [12,13,14,15,16,17,18,19,20,21,22,23]. Most of these investigated plant extracts exhibit a moderate to high inhibition efficiency in the range 55–90% in acidic media.
Crataegus oxyacantha (Hawthorn) also known as maybush, or whitehorn, is part of a genus of spiny shrubs and trees native to temperate regions in the Northern Hemisphere in Europe, Asia, and North America. It belongs to the Rosaceae family and consists of bright green leaves, white flowers, and bright red berries. Flavonoids such as vitexin, hyperoside, rutin, or vitexin-2′′-O-α-l-rhamnoside, and catechin/epicatechin derived oligomeric procyanidins are the most important constituent of Hawthorn extract [24].
Prunus Avium (sweet cherry) is geographically distributed around the world, with greater prevalence in areas with a temperate climate, which encompasses much of Europe (Mediterranean and Central), north Africa, Near and Far East, South Australia and New Zealand, and temperate zones of the American continent (USA and Canada, Argentina and Chile) [25]. Sweet cherries have been reported to contain various phenolics and anthocyanins which contribute to total antioxidant activity [26].
This work aims to explore the influence of Crataegus oxyacantha and Prunus Avium, leaf extracts on the corrosion of mild steel in hydrochloric acid using electrochemical techniques.
Experimental studies
Solution preparation
0.5 M HCl solutions were prepared by dilution of 37% concentrated grade acid, from Scharlau chemical industries using distilled water.
Extraction procedure
Crataegus oxyacantha and Prunus Aviums stock solutions were obtained by drying the plant leaves for 2 h in an oven at 80 °C and grinding to powdery form. A 10 g sample of the powder was refluxed in 100 mL distilled water for 1 h. The refluxed solutions were filtered to remove any contamination. The concentrations of the stock solutions were determined by evaporating 10 mL of the filtrates and weighing the residues. Prior to each experiment, an appropriate volume of 4 M HCl is added to an appropriate volume of the stock solution of plant leaf extract and double distilled water to obtain a solution of 0.5 M HCl solutions and the required concentration of the extract.
Electrochemical studies
Electrochemical impedance (EIS) and polarization measurements were done using frequency response analyzer (FRA)/potentiostat supplied from ACM instruments (UK). The frequency range for electrochemical impedance spectroscopy (EIS) measurements was 0.1 to 96 × 103 Hz with applied potential signal amplitude of 10 mV around the rest potential. The data were obtained in an electrochemical cell of three-electrode mode; platinum wire and saturated calomel electrodes (SCE) were used as counter and reference electrodes. The mild steel used for constructing the working electrode was of the following chemical composition (wt%) (C: 0.164, Mn: 0.710, Si: 0.260, S: 0.001, P: 0.005 and Fe: 96.2). The steel plate of cylindrical shape was encapsulated in Teflon in such a way that only one surface was left uncovered. The exposed area (0.7853 cm2) was mechanically abraded with a series of emery papers of variable grades, starting with a coarse one and proceeding in steps to the finest (800) grade. Before polarization and EIS measurements, the working electrode was left for 20 min to attain the open circuit potential in the used solution. Linear polarization measurements (LPR) were carried out at a sweep rate of 10 mV min−1 within a potential range of ± 10 mV from the rest potential. Polarization curve measurements were obtained at a scan rate of 30 mV/min starting from cathodic potential (Ecorr = − 250 mV) going to anodic direction (Ecorr = + 250 mV). All the measurements were done at 30 ± 0.1 °C using WiseCircu water bath (Germany) in solutions open to the atmosphere under unstirred conditions.
To obtain the activation parameters, the measurements were carried out at 30–60 °C. To test the reliability and reproducibility of the measurements, duplicate experiments were performed, under the same conditions, in each case and found to be within 2% error.
Ultra-violet spectroscopy (UV) and FTIR analysis
FTIR analysis of the plant extracts was carried by FTIR 8400S Shimadzu in the spectral region between 4000 and 400 cm−1. The optical studies were measured using the ultraviolet-visibleV-670 that measures the absorption spectra at a wavelength of 800–200 nm at room temperature.
Results and discussion
Open circuit potential measurements (OCP)
Figure 1 reveals that the OCP of mild steel in 0.5 M HCl solutions in the absence and presence of 0.4 g L−1 leaf extracts is attained after 20 min of immersion. It is clearly observed that the variation of OCP after 20 min is within 2 mV min−1, indicating that the mild steel electrode reached its equilibrium state at this time. The OCP of mild steel electrode was shifted towards less negative values in the presence of the leaf extracts. Such positive shift of the corrosion potential indicates the influence of these extracts on the anodic process [27].
Potentiodynamic polarization data measurements
The potentiodynamic polarization curves of mild steel shown in Fig. 2 indicate that the addition of leaf extracts suppresses both anodic metal dissolution and the cathodic hydrogen evolution reactions indicating that they could be classified as mixed type inhibitors.
The electrochemical parameters including the corrosion current density (icorr) that is obtained from the intersection of the extrapolation of anodic and cathodic Tafel lines together with percentage of inhibition efficiency (%P) are given in Table 1. The %P was calculated from polarization measurements using the relation: %P = [(i0 − i)/i0] × 100
where i0 and i are the corrosion current densities in the absence and the presence of plant leaf extracts, respectively.
The displayed data showed that icorr decreases with increasing Crataegus oxyacantha and Prunus Avium leaf extracts concentrations accompanied with an increase in %P. The slight variations in anodic and cathodic Tafel slopes, βa and βc, in the presence of these extracts indicate that the inhibiting action is taking place the simple blocking of existing cathodic and anodic sites on the metal surface [28]. The studied leaf extracts could be classified as pickling type inhibitors since they approximately have no effect on the corrosion potential (Ecorr) [29].
Electrochemical impedance spectroscopy results
The Nyquist plots shown in Fig. 3 consist of depressed semicircles of capacitive type signifying that the dissolution process of mild steel occurs under activation control [30]. The depressed capacitive loop is ascribed to dispersion effects, which have been attributed to roughness and inhomogeneities on the surface during corrosion [28, 31].
The obtained Nyquist impedance plots were examined by fitting the experimental data to a simple equivalent circuit model, Fig. 4, which includes the solution resistance Rs and the constant phase element (CPE) together with the charge transfer resistance element Rct. The Rct value is a measure of electron transfer across the surface and is inversely proportional to corrosion rate.
To compensate for non-homogeneity in the system, the capacitances were implemented as a constant phase element (CPE) that is defined by two values, the non-ideal double layer capacitance and n.
The impedance, Z, of CPE is presented by
where i = (− 1)1/2, ω is the frequency in rad s−1, ω = 2Πf, and f is the frequency in Hz. If n equals 1, the value of Q present in the above equation is identical to that of ideal capacitor C. Then, the ZCPE = (iωC)−1. In this case, the Q that is equal to C has units of capacitance, i.e., µF/cm2, and represents the capacity of the interface. However, for a non-homogeneous system, where n values are different from 1, Q is equal to the CPE admittance (Y0) and has units of µsn/Ω cm2. In this case, the system shows behavior that has been attributed to surface heterogeneity or to continuously distributed time constants for charge transfer reactions [32,33,34,35]. The double layer capacitance (Cdl) could be calculated using the following equation [36]:
The fitting of the spectrum to the equivalent circuit model permits the evolution of the elements of the circuit analog. The experimental and computer fitting results of the Nyquist plot of 0.3 g L−1 Crataegus oxyacantha in 0.5 M HCl at 30 °C are demonstrated in Fig. 5.
The percentage inhibition efficiency (%P) can be obtained from impedance measurements according to the equation:
where Rct0 and Rct are the values of the charge transfer resistance (Ω cm2) in the absence and the presence of leaf extracts, respectively.
The values of electrochemical impedance parameters obtained from fitting the experimental data to the used equivalent model and the values of %P are presented in Table 2. The data indicate that increasing plant leaf extracts concentrations increases the charge transfer resistance and a decrease in the ideal double layer capacitance Cdl values. Such reduction is due to the increase in the thickness of the electrical double layer suggesting that the leaf extracts’ molecules act by adsorption at the metal/solution interface [31, 37].
Linear polarization resistance (LPR)
The inhibition efficiency (%P) was calculated from polarization resistance (Rp) values obtained from LPR measurements using the following equation:
where Rp0 and Rp are the values of the polarization resistance (Ω cm2) in the absence and the presence of leaf extracts, respectively.
Figure 6 shows the variation of %P obtained from Rp values as function of concentration of Crataegus oxyacantha and Prunus Avium leaf extracts in 0.5 M HCl at 30 °C. It is clearly observed that as the concentration of Crataegus oxyacantha and Prunus Avium leaf extracts increases, the values of %P increase up to 55–60%.
The values of %P are in quite good agreement with the results obtained previously from potentiodynamic polarization curves and impedance measurements
Adsorption isotherms
To discuss adsorption isotherms, the degrees of surface coverage values were obtained from AC impedance measurements using equation (θ = %P/100). Theoretical fitting of different isotherms, Langmuir, thermodynamic-kinetic model and Florry–Huggins isotherm was tested to describe the mode of inhibitors’ adsorption on mild steel surface.
Langmuir isotherm is given by [38]
where K is the equilibrium constant of the adsorption process, C is the inhibitor‘s concentration and the Florry–Huggins isotherm [39] which is given by
“x” is the size parameter and is a measure of the number of adsorbed water molecules substituted by a given inhibitor molecule.
The kinetic-thermodynamic model [40] is given by
where “y” is the number of inhibitor molecules occupying one active site; in other words, “1/y” is the number of surface active sites occupied by one inhibitor molecule. The binding constant K is given by
Figure 7a–c shows the application of the above-mentioned models to the results of adsorption of the used extracts on mild steel surface in 0.5 M HCl. The parameters obtained from the fitting these isotherms are depicted in Table 3.
a Application of Langmuir adsorption isotherm to the results of adsorption of Crataegus oxyacantha and Prunus Avium on mild steel surface in 0.5 M HCl. b Application of Kinetic–thermodynamic model to the results of adsorption of Crataegus oxyacantha and Prunus Avium on mild steel surface in 0.5 M HCl. c Application of Florry–Huggins model to the results of adsorption of Crataegus oxyacantha and Prunus Avium on mild steel surface in 0.5 M HCl
It was found that the experimental data fitted all the applied adsorption isotherms except Langmuir. Such observation indicates the non-ideal behavior of these plant leaf extracts. The number of active sites occupied by a single inhibitor molecule, 1/y, is greater than unity indicating that each molecule of the leaf extracts was adsorbed onto more than one active site. Thus, the adsorbed molecules are bulky [41].
Moreover, it is known that the inhibition efficiency is a function of the value of inhibitor’s binding constant Kads, which represents the strength between adsorbed species and metal surface. The large values of K clarify stronger interaction, whereas small values of K signify that the interaction is weaker [17]. Hence, according to the numerical values of K obtained from the three models, the inhibition efficiency of Crataegus oxyacantha is better than Prunus Avium.
However, the equilibrium constant K is related to the standard free energy of adsorption (ΔGads) according to the equation:
where K is the binding constant obtained from kinetic–thermodynamic model, R is the molar gas constant, T is the absolute temperature in Kelvin and 55.5 is the concentration of water in solution expressed in molar [26].
The calculated ΔGads values from kinetic–thermodynamic model were − 36 and − 31 kJ.mol−1 for Crataegus Oxycantha and Prunus Avium in 0.5 M HCl solutions, respectively. Such values are an indication of the spontaneity of the adsorption process of both leaf extracts, and the stability of the adsorbed layers on the mild steel surface in 0.5 M HCl solution. The obtained ΔGads values reveal that the adsorption takes place through physisorption mechanism [26].
Activation Parameters
Figure 8 represents the Nyquist Impedance plots for mild steel in 0.5 M HCl in the presence of 0.4 g L−1 Crataegus oxyacantha leaf extract at different temperatures. As seen, increasing the temperature decreases the size of the depressed semicircles indicating a decrease in the charge transfer resistance (Rct) and, thus, an increase in the corrosion rate. Such behavior confirms the desorption of plant extract molecules from the metal surface at elevated temperatures.
The activation parameters for mild steel in 0.5 M HCl in the absence and presence of 0.4 g L−1 plant extracts were obtained from the linear square fitting of ln(υ) and ln (υ/T) data vs. (1/T), by applying Arrhenius and transition state equations [25].
The corrosion rates (υ) were taken as the reciprocals of the charge transfer resistance (Rct) which were obtained from the Nyquist plots of different temperatures. The apparent activation energy, Ea, activation entropies, ΔS* and activation enthalpies, ΔH*, in the absence and presence of plant extracts are depicted in Table 4.
Table 4 revealed that Ea and ΔH* values increase in the presence of both plant leaf extracts, indicating a higher protection efficiency [29]. The positive values of ΔH* indicate that the formation of the activated complex is endothermic process. However, the negative value of ΔS* implies that the activated complex represents an association rather than a dissociation step. This means that a decrease in disordering takes place on going from reactants to the activated complex [26, 42].
Spectrophotometric and FTIR analysis
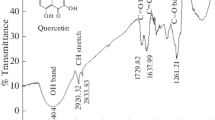
Several studies used FTIR analysis as a tool to determine functional groups present in any extract [11, 43, 44]. Figure 9a, b shows the FTIR spectra of Crataegus oxyacantha and Prunus Avium plant leaves’ extracts. IR spectrum for Crataegus oxyacantha showed absorption bands for C–H stretching vibrations, generally in the range of 2923–2800 cm−1; a broad –OH group (3420 cm−1); and a C=C vibration for aromatics (1653.83–1463.83 cm−1). Likewise bands were obtained for Prunus Avium leaf extract in addition to C=O at 1739 cm−1 that is attributed to esters. By matching these spectra with the literature in order to determine the active chemical ingredients for these plant leaves extracts under study [45, 46], it was found that catechin may be the major active chemical ingredient in Crataegus oxyacantha leaf extract, whereas methylvanillate in Prunus Avium extract.
Mechanism of inhibition
It is clearly observed from the UV spectra presented in Fig. 10 that there is no shifting in the absorption bands of plant extract (PE) in the presence of 10−3 M FeSO4. Such observation is quite an indication of the absence of [Fe-PE]2+complex and that the inhibition takes place through simple physical adsorption of extract molecules on the surface according to the following equation:
Conclusion
Crataegus oxyacantha and Prunus Avium leaf extracts acted as good corrosion inhibitors for mild steel in 0.5 M HCl solution. An excellent agreement between the inhibition efficiencies calculated using different electrochemical techniques was obtained. Such inhibition of these plant leaf extracts depends on the physical adsorption of the chemical constituents of the extract on mild steel surface rather than forming a complex with Fe2+ ions.
References
Gobara M, Zaghloul B, Baraka A, Elsayed M, Zorainy M, Kotb MM, Elnabarawy H (2017) Green corrosion inhibition of mild steel to aqueous sulfuric acid by the extract of Corchorus olitorius stems. Mater Res Express 4:046504. https://doi.org/10.3390/ma10080956
Abdel-Gaber AM, Khamis E, Hefnawy A (2011) Utilizing Arghel extract as corrosion inhibitor for reinforced steel in concrete. Mater Corros 62:1159–1162
Khadom AA, Abd AN, Ahmed NA (2017) Xanthium strumarium leaves extracts as a friendly corrosion inhibitor of low carbon steel in hydrochloric acid: kinetics and mathematical studies. SAJCE 25:13–21
Seifzadeh D, Basharnavaz H (2013) Corrosion protection of AZ91 magnesium alloy in cooling systems. Trans Nonferrous Meterol Soc China 23:2577–2584
Ashassi-Sorkhabi H, Seifzadeh D (2006) The inhibition of steel corrosion in hydrochloric acid solution by juice of Prunus cerasus. Int J Electrochem Sci 1:92–96
Belarbi N, Dergal F, Chikhi I, Merah S, Lerari D, Bachari K (2018) Study of anti-corrosion activity of Algerian L. stoechas oil on C38 carbon steel in 1 M HCl medium. IJIC 1–11
Prasanna BM, Praveen BM, Hebbar N, Venkatesha TV, Tandon HC (2016) Inhibition study of mild steel corrosion in 1 M hydrochloric acid solution by 2-chloro 3-formyl quinoline. IJIC 7:9–19
Seifzadeh D, Basharnavaz H, Bezaatpour A (2013) A Schiff base compound as effective corrosion inhibitor for magnesium in acidic media. Mater Chem Phys 138:794–802
Seifzadeh D, Bezaatpour A, Joghani RA (2014) Corrosion inhibition effect of N, N’-bis (2-pyridylmethylidene)-1, 2-diiminoethane on AZ91D magnesium alloy in acidic media. Trans Nonferrous Metals Soc China 24:3441–3451
Salah M, Lahcène L, Omar A, Yahia H (2017) Study of corrosion inhibition of C38 steel in 1 M HCl solution by polyethyleneiminemethylene phosphonic acid. IJIC 8:263–272
Abakedi OU, Ekpo VF, John EE (2016) Corrosion inhibition of mild steel by Stachytarphetaindica leaf extract in acid medium. TPCJ 3:165–171
Hijazi KM, Abdel-Gaber AM, Younes GO (2015) Electrochemical corrosion behavior of mild steel in HCl and H2SO4 solutions in presence of loquat leaf extract. Int J Electrochem Sci 10:4366–4380
Yang W, Wang Q, Xu K, Yin Y, Bao H, Li X, Chen S (2017) Enhanced corrosion resistance of carbon steel in hydrochloric acid solution by Eriobotrya japonica thunb leaf extract: electrochemical study. Materials 10:956
Abdel-Gaber AM, Abd-El-Nabey BA, Sidahmed IM, El-Zayady AM, Saadawy M (2006) Inhibitive action of some plant extracts on the corrosion of steel in acidic media. Corros Sci 48:2765–2779
Abdel-Gaber AM, Abd-El-Nabey BA, Saadawy M (2009) The role of acid anion on the inhibition of the acidic corrosion of steel by lupine extract. Corros Sci 51:1038–1042
Hijazi KM, Abdel-Gaber AM, Younes GO (2015) Influence of malus domestica and Caesalpinia bonducella leaf extracts on the corrosion behaviour of mild steel in H2SO4 solution. Int J Electrochem Sci 10:4779–4792
Abd-El-Naby BA, Abdullatef OA, Abd-El-Gabr AM, Shaker MA, Esmail G (2012) Effect of some natural extracts on the corrosion of zinc in 0.5 M NaCl. Int J Electrochem Sci 7:5864–5879
Abd-El-Nabey BA, Abdel-Gaber AM, Elawady GY, El-Houssein S (2012) Inhibitive action of some plant extracts on the alkaline corrosion of aluminum. Int J Electrochem Sci 7:7823–7839
Abdel-Gaber AM, Hijazi KM, Younes GO, Nsouli B (2017) Comparative study of the inhibitive action between the bitter orange leaf extract and its chemical constituent linalool on the mild steel corrosion in HCl. Quím Nova 40:395–401
Lebrini M, Robert F, Lecante A, Roos C (2011) Corrosion inhibition of C38 steel in 1 M hydrochloric acid medium by alkaloids extract from Oxandra asbeckii plant. Corros Sci 53:687–695
Deng S, Li X (2012) Inhibition by Ginkgo leaves extract of the corrosion of steel in HCl and H2SO4 solutions. Corros Sci 55:407–4015
Abd-El-Khalek DE, Abdel-Gaberb AM (2012) Evaluation of nicotiana leaves extract as corrosion inhibitor for steel in acidic and neutral chloride solutions. Portugaliae Electrochimica Acta 30:247–259
Wang J, Xiong X, Feng B (2013) Effect of crataegus usage in cardiovascular disease prevention: an evidence-based approach. Evidence-Based Complementary and Alternative Medicine
Bastos C, Barros L, Dueñas M, Calhelha RC, Queiroz MJR, Santos-Buelga C, Ferreira IC (2015) Chemical characterization and bioactive properties of Prunusavium L.: the widely studied fruits and the unexplored stems. Food Chem 173:1045–1053
Usenik V, Fabčič J, Štampar F (2008) Sugars, organic acids, phenolic composition and antioxidant activity of sweet cherry (Prunusavium L.). Food Chem 107:185–192
Rahal HT, Abdel-Gaber AM, Younes GO (2016) Inhibition of steel corrosion in nitric acid by sulfur containing compounds. Chem Eng Commun 203:435–445
Rahal HT, Abdel-Gaber AM, Awad R, Abdel-Naby BA (2018) Influence of nitrogen immersion and NiO nanoparticles on the electrochemical behavior of (Bi, Pb)-2223 superconductor in sodium sulfate solution. Anti-Corros Methods Mater 65:430–435
Shukla SK, Quraishi MA, Ebenso EE (2011) Adsorption and corrosion inhibition properties of cefadroxil on mild steel in hydrochloric acid. Int J ElectrochemSci 6:2912–2931
Abdel-Gaber AM, Masoud MS, Khalil EA, Shehata EE (2009) Electrochemical study on the effect of Schiff base and its cobalt complex on the acid corrosion of steel. Corros Sci 51:3021–3024
Rafiquee MZA, Khan S, Saxena N, Quraishi MA (2007) Influence of some thiadiazole derivatives on corrosion inhibition of mild steel in formic and acetic acid media. PortugaliaeElectrochimicaActa 25:419–434
Lebrini M, Robert F, Roos C (2010) Inhibition effect of alkaloids extract from Annonasquamosa plant on the corrosion of C38 steel in normal hydrochloric acid medium. Int J ElectrochemSci 5:1698–1712
Orazem ME, Tribollet B (2011) Electrochemical impedance spectroscopy (vol 48). Wiley, Hoboken
Macdonald JR (1985) Generalizations of ‘‘universal dielectric response’’ and a general distribution of activation energies model for dielectric and conducting systems. J Appl Phys 58:1971–1978
Nezamdoust S, Seifzadeh D, Rajabalizadeh Z (2018) PTMS/OH-MWCNT sol-gel nanocomposite for corrosion protection of magnesium alloy. Surf Coat Tech 335:228–240
Rajabalizadeh Z, Seifzadeh D (2017) Application of electroless Ni-P coating on magnesium alloy via CrO3/HF free titanate pretreatment. Appl Surf Sci 422:696–709
Ghanbari A, Attar MM, Mahdavian M (2010) Corrosion inhibition performance of three imidazole derivatives on mild steel in 1 M phosphoric acid. Mater Chem Phys 124:1205–1209
Nwabanne JT, Okafor VN (2012) Adsorption and thermodynamics study of the inhibition of corrosion of mild steel in H2SO4 medium using vernoniaamygdalina. JMMCE 11:885–889
Langmuir I (1916) The constitution and fundamental properties of solids and liquids. J Am Chem Soc 38:2221–2295
Flory PJ (1942) Thermodynamics of high polymer solutions. J Che. Phy. 10:51–61
El-Awady AA, Abd-El-Nabey BA, Aziz SG (1992) Kinetic-thermodynamic and adsorption isotherms analyses for the inhibition of the acid corrosion of steel by cyclic and open-chain amines. J Electrochem Soc 139:2149–2154
Karthikaiselvi R, Subhashini S (2014) Study of adsorption properties and inhibition of mild steel corrosion in hydrochloric acid media by water soluble composite poly (vinyl alcohol-o-methoxy aniline). J Assoc Arab Univ Basic Appl Sci 16:74–82
Manimegalai S, Manjula P (2015) Thermodynamic and adsorption studies for corrosion inhibition of mild steel in aqueous media by sargasamswartzii (Brown algae). J Mater Environ Sci 6:629–1637
Kolo AM, Ahmed A, Ajanaku IK, Ameh PO (2017) Electrochemical study of the corrosion inhibition of Delonix regia for mild steel in sulphuric acid medium. J Indus Environ Chem 1:15–21
Hassan KH, Khadom AA, Kurshed NH (2016) Citrus aurantium leaves extracts as a sustainable corrosion inhibitor of mild steel in sulfuric acid. South Afr J Chem Eng 22:1–5
Ben Hmamou D, Salghi R, Zarrok H, ZarroukAbdelkader Hammouti B, El Hezzat M, Bouachrine M (2012) Temperature effects on the corrosion inhibition of carbon steel in acidic solutions by alizarin red. Adv Mater Corros 1:36–42
Sanz M, Cadahía E, Esteruelas E, Muñoz AM, Fernández De Simón B, Hernandez TERESA, Estrella I (2010) Phenolic compounds in cherry (Prunusavium) heartwood with a view to their use in cooperage. J Agric Food Chem 58:4907–4914
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Al-Moghrabi, R.S., Abdel-Gaber, A.M. & Rahal, H.T. A comparative study on the inhibitive effect of Crataegus oxyacantha and Prunus avium plant leaf extracts on the corrosion of mild steel in hydrochloric acid solution. Int J Ind Chem 9, 255–263 (2018). https://doi.org/10.1007/s40090-018-0154-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40090-018-0154-3