Abstract
The current study was designed to give more knowledge and helping to exploit the leaves of the plants L. stoechas collected in Tlemcen region in the west of Algeria by determining the anti-corrosive activity of its essential oil. The inhibitory efficiency of the essential oil of the plant obtained by hydrodistillation and characterized by GC and GC/MS was studied using gravimetric and electrochemical methods (polarization curves and electrochemical impedance). The effect of temperature on the corrosion behavior of the steel and the inhibitory efficiency was studied in a temperature range of 303–323 K at 2 g/L. The surface morphology of the samples immersed in 1 M HCl for 24 h, before and after adding inhibitor at (2 g/L at 30 °C) was analyzed by scanning electron microscope (SEM) Quanta 250 with tungsten filament from the company FEI. Results show that the addition of the essential oil of the plant to the medium induces a diminish in the rate of corrosion and augmentation in the inhibitory efficiency of the oil. We established that the inhibition efficiency increase with concentration of the essential oil of lavender to attain 76.19% at 2 g/L. Polarization curves revealed that lavender oil react as a mixed-type inhibitor. EIS spectra exhibit one capacitive loop and confirm the inhibitive ability, and the changes in impedance parameters were indicative of adsorption of essential oil of lavender on the metal surface. The thermodynamic parameters indicate that the adsorption of the molecules of the oil takes place according to the Langmuir isotherm in the corrosive medium studied and that they are physisorbed on the metal surface. The analysis of the surface by electron microscopy demonstrates the absence of surface attack patterns in the presence of the oil. The results obtained from different tested techniques were in good agreement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carbon steel is a major construction material and is widely used for downhole tubular, casings, flow lines and transmission pipelines in petroleum industry [1, 2]. It is also used as a structural component in ships manufacture. Acidic solutions are extensively used as aggressive solutions in acid processes of pickling, cleaning, descaling, and oil well acidization [1,2,3]. During descaling processes that remove rust and mill scale formed during production, steel and other alloys are immersed in acidic solutions. Due to the aggressiveness of acids, steel corrodes severely during these processes particularly with the use of hydrochloric acid, which results in terrible waste of both resources and money [1, 2]. The damage due to corrosion can be decreased using of corrosion inhibitors.
Organic inhibitors represent a very important group of corrosion inhibitors. The effectiveness of the organic inhibitors is related to the structure, the concentration and the chemical properties of the layer formed on the specified conditions. The action of an organic inhibitor is the result of its adsorption on the surface of the material. Most of these inhibitors have in their structure mainly nitrogen, sulfur or oxygen atoms [4,5,6]. Inhibitors that contain sulfur are more effective than those that contain nitrogen, because sulfur is a better electron donor than nitrogen [7]. Unfortunately, most of these compounds are not only expensive but also toxic to living things. These toxic effects have led to the use of natural products as anti-corrosion agents which are ecological and harmless. Very recently, many ecological corrosion-inhibiting alternatives have been developed, ranging from rare earths to organic compounds [8]. Plant extracts which are considered as green inhibitors, have become important and environmentally acceptable, easily accessible and renewable sources for a wide range of inhibitors. They are rich sources of ingredients that have very high inhibition efficiency [9]. The yield of these natural products as well as the corrosion inhibition capacity changes significantly depending on the part of the plant utilized and its locations [10]. Several studies have been performed on the protection of metals from corrosion by essential oils. The oil of the seeds of Prickly Pear (Opuntia ficus-indica L.) was used by Ben Hmamou et al. [11] versus the corrosion of C38 steel in acidic medium. The use of the essential oil of Mentha spicata L. by Znini et al. [12], revealed a very important inhibitory effect against steel corrosion. Baumhara et al. [13] reported that the oil of Artemisia mesatlantica is a potential green corrosion inhibitor using aerial parts of the plant, Clove oil was studied as inhibitor for iron in acidic medium [14], Abdulwahab et al. [15] investigated the anti-corrosion effect of Avogadro natural oil on mild steel in hydrochloric acid solutions, Afia et al. [16] indicate that Argan oil gives adequate protection to the steels in a medium containing 1 M HCl. More recently, Lavender oil has been found to be not harmful to the environment as corrosion inhibitor, Halambek et al. [17] have carried out a study on the influence of Lavandula angustifolia L. on corrosion of Al–3 Mg alloy in 3% NaCl solution, the results showed an efficiency of 99% for a concentration of 20 ppm at 25 °C. The study by Znini et al. [18] on the uses of Lavandula multifida L. oil as a corrosion inhibitor in 0.5 M H2SO4 medium, showed that the oil exhibited a high inhibition efficiency of 72.2% at 2 g/L of oil at 25 °C, the usage of L. stoechas oil, abstracted from leaves as corrosion inhibitor of stainless steel in H3PO4 solution was elaborated by Boudalia et al. [19]. The inhibitory efficiency was 87.3% at 1.2 g/L at 25 °C. Zerga et al. [20] have got an efficiency of 90% for 2 g/L using lavender oil as inhibitor. In Algeria, no study has been conducted on the use of the essential oil of L. stoechas species as corrosion inhibitor.
Lavandula stoechas used in the present study is a kind of plant that produces flowers, from the family Lamiaceae (30–100 cm tall), growing naturally in Mediterranean land characterized by hot, dry and sunny conditions in alkaline soils. Essential oil of L. stoechas have been used to treat depression, diabetes, epilepsy, headaches, it posses sedative properties and anti-inflammatory, antimicrobial, antifungal, and antioxidant activities [21]. The composition of the essential oil of the plant has been investigated and the oil has been found to be rich in camphor, it has been analyzed by use of both GC and GC–MS. The results revealed 32 constituents, among which camphor (37.4%) and fenchone (30.3%), were the most abundant. camphor resemble to wax in consistency or appearance, it is flammable, white or transparent solid with a robust odor. It is a terpenoid with chemical formula C10H16O. We aim in this paper to study the inhibitive action of essential oil of lavender on the corrosion behavior of steel in 1 M HCl using a gravimetric method and electrochemical techniques. Characterization of the steel surface before and after film formation was also carried out.
Experimental
Extraction of L. stoechas essential oil
Aerial part of lavandula plant was harvested in Beni Snous locality in the west Tlemcen (Algeria). Relative GPS coordinates of the station are 34°46′30.6′′N latitude and 1°26′18.2′′W longitude. Essential oil was obtained by hydrodistillation for 3 h using Clevenger-type apparatus. To analyze the chemical composition of the oil, it was dehydrated over anhydrous sodium sulfate and then stored in sealed glass vials at 4–5 °C.
Gas chromatography analysis
The gas chromatographic analysis was performed using a Perkin–Elmer apparatus equipped with a dual flame ionization detection system and a fused-silica capillary columns (60 m × 0.22 mm I ID, film thickness 0.25 µm), Rtx-1 polydimethylsiloxane). The oven temperature was varied from 60 to 230 °C at 2 °C/min. The final temperature was maintained at 230 °C for 35 min. Injector and detector temperatures were maintained at 280 °C. Essential oil was injected in the split mode (1/50), using helium as the carrier gas (1 mL/min); the injection volume was 0.2 µL. Retention indices (I) of the compounds were determined relative to the retention times of the series of n-alkanes (C5–C30) with linear interpolation, using the Van den Dool and Kratz equation and software from Perkin–Elmer.
Gas chromatography mass spectrometry (GC–MS)
The GC–MS analysis was carried with same conditions described as those of GC method, using a Perkin–Elmer Turbomass detector (quadrupole), coupled to a Perkin–Elmer Autosystem XL, equipped with the fused-silica capillary columns Rtx-1 (ion source temperature 150 °C; energy ionization 70 eV). EI mass spectra were acquired over the mass range 35–350 Da (scan time 1 s).
Corrosion measurement
Gravimetric corrosion tests
Corrosion tests were performed on carbon steel (C38) with chemical composition as follows (in Table 1):
We prepared the aggressive 1 M HCl solution by diluting a 37% HCl solution using bidistilled water. The gravimetric measurements were carried out using rectangular carbon steel samples with dimensions of: 20 mm × 10 mm × 2 mm. Before measurement, the carbon steel samples were polished with 400, 600, and 1200 grades of emery paper. They were cleaned with acetone and then rinsed with distilled water and dried. After weighing, each steel sample C38 was immersed in the acid solution (100 mL) in nonexistence and existence of different concentrations of the essential oil of lavender. The immersion time was 4 h at a temperature of 30 °C. Each gravimetric measurement is the average of at least three tests under the same conditions. These data are used to calculate the corrosion rate in milligrams per square centimeter per hour.
Electrochemical measurements
Electrochemical impedance spectroscopy (EIS) is an analytical method used to examine the interface between a material, presented as an electrode, and predefined solution. The amplitude of the sinusoidal voltage applied to the abundant potential is 10 mV peak-to-peak at frequencies between 10 and 20 mHz. The equivalent electric circuit was determined according the Zview program.
Measurements of EIS and potentiodynamic polarization were performed using Metrohm Autolab which containing a PGSTAT 302 N potentiostat, a computer and Nova 2.0 software. For the electrochemical tests, we used a three-electrode cell, double-wall thermostat with a capacity of 500 mL. The auxiliary electrode is a platinum electrode; the reference electrode is a saturated calomel electrode (SCE) with a Lugging capillary, whose end is placed near to the working electrode to minimize the influence of the ohmic drop. The working electrode is in the form of a disc with a surface S = 0.5 cm2 introduced into a sample holder of polytetrafluoroethylene arranged opposite the auxiliary electrode.
The AC impedance measurements were performed at corrosion potentials (Ecorr) over a frequency range of 10–20 mHz, with a signal amplitude perturbation of 10 mV. Nyquist plots were obtained from the results of these experiments. Measurements were conducting after maintaining the working electrode at its circuit potential for 60 min.
Before each measurement the electrode was polished with emery paper and immediately transferred into the test solution.
The potentiodynamic current–potential curves were recorded by changing the electrode potential automatically from − 800 to − 250 mV with scanning rate of 0.5 mV/s, under static on the same electrode without any surface treatment. Corrosion current densities were determined by extrapolating the cathodic Tafel regions from the potentiodynamic polarization curves to the corrosion potential. All experiments were carried out in freshly prepared solution at constant temperatures. The fixed temperatures are maintained using a water bath equipped with a Julabo thermostat.
All reagents used are very pure and analytical grade from Sigma-Aldrich. They were used without further purification.
Surface characterization
The surface morphology of the samples before and after adding of the essential oil of lavender inhibitor at 2 g/L in the medium 1 M HCl after 1 day of immersion was observed by SEM Quanta 250 with tungsten filament from the company FEI.
Results and discussion
Lavandula. stoechas oil analysis
The analysis of L. stoechas oil allowed the identification of 32 components, representing 94.8% of the total content. The oil showed a large presence of monoterpene (93.7%) distributed in oxygenated monoterpene (85.6%) and hydrocarbon monoterpene (8.1%). Little quantity of hydrocarbon sesquiterpene (0.2%) and oxygenated sesquiterpene (0.8%) was also detected. The main constituents of L. stoechas essential oil were camphor (37.4%) followed by fenchone (30.3%) and 1,8-cineole (7.6%). The compounds identified and the compositions of the essential oil are reported in Table 2.
Corrosion measurement
Weight loss measurements
Before starting the electrochemical study, it is necessary to estimate the metal–oil system behavior in the presence of an aggressive solution. However, the weight loss measurement is a first approach to use to define the inhibition of a metal corrosion in an electrolytic solution. This method consists of measuring the samples mass loss, surface S during the immersion in a corrosive solution.
The corrosion rate is calculated as follows (Eq. 1):
where Wcorr is the corrosion rate. It is expressed in mg/cm2/h, S is the total surface area of the specimen, w1 and w2 are the weights before and after exposure to the test solution, respectively, t is the immersion time.
The percentage of inhibition efficiency P (%) was calculated using the following equation:
where W0 and Winh are the corrosion rates of steel in the nonexistence and existence of inhibitor, respectively.
Table 3 shows the inhibition efficiency of carbon steel corrosion in 1 M HCl solution, at different concentrations of the inhibitor.
As shown in Table 3, corrosion rate decreases, while the inhibition efficiency increases with increasing inhibitor concentration reaching a maximum value of 61.97% at a concentration of 2 g/L. This can be attributed to the adsorption of the inhibitor molecule at the metal surface. So, the adsorption of the inhibitor at the metal/solution interface is the first step in the mechanism of inhibition in aggressive medium as demonstrated by Merah et al. [22].
Potentiodynamic polarization study
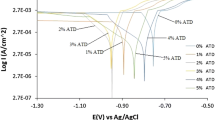
For a better evaluation of the efficiency of the proposed green inhibitor, the polarization technique was investigated. The anodic and cathodic polarization curves of the carbon steel electrode, at different concentrations of oil, in the presence of 1 M HCl, at 30 °C are shown in Fig. 1.
The values of Icorr, the corrosion potential (Ecorr), cathodic Tafel slope (bc), and inhibition efficiency P (%) are given in Table 4.
The Icorr values were used to calculate the inhibition efficiency, using the following equation:
where Icorr and Icorr(inh) are the corrosion current density values in the presence and absence of inhibitor, respectively, determined by extrapolation of cathodic Tafel lines to the corrosion potential.
The examination of Fig. 1; Table 4 shows that both anodic and cathodic corrosion reactions of C38 steel electrode were inhibited after adding the green inhibitor to the corrosive medium (1 M HCl). This inhibition is increased by increasing the inhibitor concentration from 0.25 to 2 g/L and achieve an efficiency of 65.12% (Table 4). This behavior indicates that the addition of the inhibitors reduces the dissolution reaction at the anode (carbon steel) and also delays the reduction of hydrogen ions at the cathode (hydrogen evolution reaction) [23, 24].
The values of corrosion potential of carbon steel in the acidic solution in the presence of inhibitor change slightly compared to the corrosion potential in the presence of acid sole. This observation clearly shows that the molecules of essential oil can be classified as a mixed-type inhibitor. The addition of L. stoechas oil to the corrosive medium leads to a decrease in corrosion current densities of steel (Icorr) and therefore an increase in polarization résistance (Table 4). This result is due to the anti-corrosive action of the proposed oil. Interestingly, the electrochemical results are in agreement with those acquired from weight loss measurements.
Electrochemical impedance spectroscopy characterizations
The EIS was applied to evaluate the whole process of the metal corrosion in the presence of L. stoechas oil, used as inhibitor. It can provide important information about the surface changes of the metal after modification with the oil. For a better understand, measurements were performed in 1 M HCl solution, after immersion of 1 h at free open potential at 30 °C. The steel C38 was tested at different concentration of L. stoechas oil.
Impedance diagrams obtained for frequencies ranging from 10 to 20 mHz at open circuit potential are shown in Fig. 2. These graphs consist of a main capacitive loop in the form of a semicircle whose center is located below the axis of the reals. This type of diagram generally indicates that the corrosion reaction is controlled by a process of transfer of charges on a heterogeneous and irregular solid surface electrode. We note also the presence of a single capacitive arc. Such behavior shows that the presence of the inhibitors does not alter the mechanism of dissolution of the steel in HCl.
The EIS spectra are adjusted by the equivalent circuit illustrated in Fig. 3. This circuit employed allows calculating of both solution resistance (Rs) and charge transfer resistance (Rt). It is composed by:
A charge transfer resistor (Rt) corresponding to the electrochemical reactions taking place at the interface, and a constant phase element (CPE) [25,26,27], used to account for the frequency dispersion via of the coefficient (n). The impedance of such element is [28]:
with
where Q is a proportionality factor (real constant) and Cdl is the double layer capacitance. The coefficient n has a mean of phase shift; it comprised between 0 and 1 and in a few ways represents a measure of the inhomogeneities of the surface, J is the imaginary number, and w is the angular frequency.
Table 5 includes the impedance parameter values determined by modeling, using the electric circuit equivalent proposed in Fig. 3. The corrosion-inhibiting efficiency of steel is also listed in this table. It is calculated from the relation [29]
where Rt is the charge transfer resistance in the case of steel immersed in corrosive solution, and Rt′ in the presence of oil inhibitor.
The analysis of Table 5 let us conclude that the addition of L. stoechas oil enhances the values of Rt and reduces the Cdl values. These results are related to the mechanism of adsorption of the inhibitor. Indeed, during the immersion process of the electrode steel in the protective solution, the molecules of inhibitor are adsorbed on the metal surface. The film thus formed serves as a barrier which would prevent the corroding ions of the electrolyte from reaching the surface of the electrode, which protects against corrosion. The calculated values of n are found to be within the range 0.85–0.92 which explain a type of non-homogeneity due to the presence of chloride ion that turn the steel surface rough or porous. The inhibitory efficiency increases with the concentration of the inhibitor to reach 76.19% at 2 g/L. The values obtained confirm those previously determined by gravimetric and potentiodynamic polarization.
Kinetics–thermodynamics parameter
The inhibition of corrosion of metals by the essential oil of a plant containing different chemical families is explained by the adsorption of its organic compounds. The physical or chemical nature of adsorption depends on several parameters, such as the nature of the metal and its charge, the chemical structure of organic molecules as well as the type of the electrolyte. The surface coverage (θ) for different concentrations of L. stoechas oil in acid medium is evaluated by adopting gravimetry as study methods, we can write [4, 27, 29]
where W0 is the corrosion rate in uninhibited acid and Winh is the corrosion rate in inhibited acid. Assuming that the adsorption of the inhibitor follows the Langmuir adsorption isotherm, the surface coverage of the metallic surface is given by the relationship
where K is the adsorption coefficient or the equilibrium constant of the adsorption process and C is the concentration of inhibitor. Rearranging this equation gives
Figure 4 shows that the variation of the ratio \(\frac{C}{\theta }\) depending on the inhibitor concentration C using the gravimetric measurements is linear (R2 = 0.99557). This indicates that the adsorption of molecules of lavender oil on the surface of carbon steel follows the Langmuir isotherm. Note that we have simulated this data (gravimetric measurement) with various adsorption isotherms including Frumkin, Temkin and Freundlich. The best fit was found in the case of Langmuir isotherm which approved our suggestion. The slight deviation of the value of the slope from the unity (slope = 1.37) is attributed to the existence of interactions between adsorbed species on the metal surface which corresponds to the observed physical adsorption mechanism [30, 31]. The adsorptive equilibrium constant (K) value is K = 1.74 L/g. The free energy values of adsorption (ΔG°ads) of lavender oil on carbon steel surface were calculated using Eq. (10) [32].
where R is the universal gas constant (8.314 J/K mol), T is the absolute temperature and C (sol) is the concentration of water (1000 g/L). The ∆Gads value calculated is − 18.79 kJ/mol. This negative value informs us about the spontaneity of the adsorption process and the stability of the film adsorbed on the surface of the steel. Generally, the values of ∆Gads up to − 20 kJ/mol signify physisorption, which is consistent with electrostatic interaction between charged molecules and a charged metal. The values around − 40 kJ/mol or higher involve charge sharing or transfer from the inhibitor molecules to the metal surface to form a coordinate type of bond (chemisorptions) [33, 34]. In our case, the value of ΔGads found (− 18.79 kJ/mol), suggesting that the inhibitor is physisorbed on the surface of the steel.
The likely mechanism can be explained on the basis of the adsorption process and the structure of components in the essential oil. The inhibition can be due to the adsorption of photochemical components present in the oil through oxygen atoms on the metal surface. We can assumed that the adsorption phenomenon may be made by camphor as the principal constituent of the essential oil of L. stoechas, but as the natural oil contains so many components, the inhibitory action may be due to synergistic intermolecular of the active molecules of this oil.
Effect of temperature
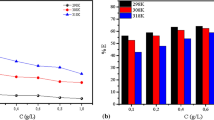
The stability of a corrosion inhibitor in an aggressive medium and at given operating temperatures is very important for its application. To elucidate the mechanism of inhibition of oil, we have examined the influence of temperature on its inhibitory efficacy. We conducted gravimetric measurements on carbon steel C38 in 1 M HCl in the nonexistence and presence of 2 g/L of L. stoechas oil at different temperature and 4 h immersion period. The values of the corrosion rate (W) and the percentage of inhibitory efficiency P (%) calculated are given in the following Table.
Examination of Table 6 revealed that the corrosion rate increases both in the uninhibited and in the inhibited acid solution with the rise of temperature. In the existence of inhibitor, the corrosion rate diminishes. P (%) increases with temperature. This confirms the chemical character of the adsorption process.
Figure 5 presents the Arrhenius plots of the logarithm of the corrosion rate vs 1/T, for 1 M solution of hydrochloric acid, without and with addition of L. stoechas oil.
In acidic solution, the corrosion rate is related to temperature by Arrhenius equation:
where W0, W are the corrosion rates of steel with no and with addition of inhibitor, in that order.
Ea, E′a are the activation energies in the nonexistence and presence of inhibitor; respectively, and K′ are constants.
The variation of the logarithm of the corrosion rate as a function of 1/T presents straight lines showing that the Arrhenius law is respected.
Activation energies are calculated from the slopes of the Arrhenius lines (Fig. 5). The values obtained of Ea are 82 and 24 kJ/mol for free acid and with the addition of oil, respectively. We remark that the presence of inhibitor causes a change in the values of apparent activation energy. These alterations may be due to the change in the mechanism of the corrosion process in the existence of adsorbed inhibitor molecules [35, 36]. The lower value of the activation energy of the process in an inhibitor presence when compared to that in its absence is attributed to its chemisorption [24].
Recall also that Szauer et al. [37, 38] consider that the low activation energy of the corrosion process in the presence of inhibitor compared to that in its absence is attributed to chemisorption unlike physical adsorption, which is usually accompanied by an increase in Ea in the presence of inhibitors.
In addition from the transition state equation [30]:
where W is the corrosion rate, R is the universal gas constant, \(\Delta H_{\text{a}}^{ \circ }\) the enthalpy of activation and T the absolute temperature. We can plot ln (w/T) = f (1/T) as shown in Fig. 6.
The \(\Delta H_{\text{a}}^{ \circ }\) values were determined from the slopes of these plots. The calculated values of \(\Delta H_{\text{a}}^{ \circ }\) are 79 kJ mol in the absence of the inhibitor and 21 kJ/mol in its presence. We remark that (\(\Delta H_{\text{a}}^{ \circ }\)) value for dissolution reaction of steel in 1 M HCl in the presence of lavender oil is less (21 kJ/mol) than that in its absence (79 kJ/mol). The positive sign of ∆Ha shows the endothermic nature of L. stoechas oil adsorption on the surface of the steel [1], suggesting that the dissolution of steel is slow in the presence of inhibitor [30].
Surface morphology
The scanning electron microscopy (SEM) micrographs (Fig. 7a, b) of the surfaces of C38 steel samples were taken at same magnification to see the changes that occurred during corrosion process in the absence and presence of lavender essential oil at 2 g/L during 24 h at 30 °C. The SEM image (Fig.7a) of the steel specimen in the absence of the inhibitor shows an irregular and damaged surface owing to rapid corrosion attack. However, a relatively smoother and less corroded morphology of steel surface can be observed in the presence of inhibitor (Fig.7b). This may be intercepted by the adsorption of molecules of this inhibitor on the electrode surface. It is thought that the molecules of inhibitor depress the corrosion by the formation of an adherent deposit on the electrode surface limiting the access of the electrolyte to the surface of the steel. This result confirms those from weight loss and electrochemical measurements.
Conclusions
The present work was devoted to the study of the inhibition of corrosion of C38 steel in 1 M hydrochloric acid by the presence of essential oil of the lavender plant, biodegradable and environmentally friendly oils are potential candidates for the protection of steels in acid environment. The evaluation of the inhibitory potency of this oil on the corrosion of C38 steel in 1 M HCl medium was carried out by various techniques, such as gravimetry, polarization curves and electrochemical impedance spectroscopy. A detailed study of the mechanism of inhibition led us to associate other methods of surface characterization, as SEM, to these usual techniques. The results show that:
-
L. stoechas oil acts as a good corrosion inhibitor for carbon steel in 1 M HCl solution.
-
The oil is a mixed-type inhibitor whose inhibitory potency increases with concentration.
-
The increase in temperature induces an increase in inhibitory activity.
-
The adsorption of the oil on the metal surface follows Langmuir isotherm.
-
The corrosion inhibition is probably due to the adsorption of L. stoechas on the metal surface and blocking its active sites by the phenomenon of adsorption.
-
The determination of different adsorption parameters proves the spontaneity of the process and the stability of the adsorbed layer.
-
SEM technique reveals that the inhibitor molecules form a good protective film on the steel surface and confirm the result obtained by gravimetric and electrochemical methods.
-
The gravimetric method, polarization curves and impedance measurement are in good agreement
References
Yadav M, Behera D, Kumar S, Sinha RR (2013) Experimental and quantum chemical studies on corrosion inhibition performance of benzimidazole derivatives for mild steel in HCl. Ind Eng Chem Res 52:6318–6328
Kumar S, Sharma D, Yadav P, Yadav M (2013) Experimental and quantum chemical studies on corrosion inhibition effect of synthesized organic compounds on N80 steel in hydrochloric acid. Ind Eng Chem Res 52:14019–14029
Banerjee S, Srivastava V, Singh MM (2012) Chemically modified natural polysaccharide as green corrosion inhibitor for mild steel in acidic medium. Corros Sci 2012(59):35–41
Li X, Shuduan D, Hui F (2010) Blue tetrazolium as a novel corrosion inhibitor for cold rolled steel in hydrochloric acid solution. Corros Sci 52:2786–2792
Fouda AS, Abdallah AM, Al-Ashrey SM, Abdel-Fattah AA (2010) Some crown ethers as inhibitors for corrosion of stainless steel 304 in aqueous solution. Desalination 250:538
Abdallah M, Zaafarany I, Khairou KS, Emad Y (2012) Natural oils as corrosion inhibitors for stainless steel in sodium hydroxide solutions. Chem Technol Fuels Oils 48:234
Loto RT, Loto CA, Popoola API (2012) Corrosion inhibition of thiourea and thiadiazole derivatives: a review. J Mater Environ Sci 3:885–894
Subir P, Ishita K (2016) Corrosion inhibition of carbon steel in acidic environment by papaya seed as green inhibitor. J Bio Tribo Corros 2:6
Mo S, Luo HQ, Li NB (2016) Plant extracts as green corrosion inhibitors for steel in sulphuric acid. Chem Pap 70:1131–1143
Khadraoui A, Khelifa A, Hadjmeliani M, Mehdaoui R, Hachama K, Tidu A, Azari Z, Obot IB, Zarrouk A (2016) Extraction, characterization and anti-corrosion activity of Mentha pulegium oil: weight loss, electrochemical, thermodynamic and surface studies. J Mol Liq 216:724–731
Benhamou D, Salghi R, Bazzi Lh, Hammouti B, Al-Deyab SS, Bammou L, BazziL Bouyanzer A (2012) Prickly pear seed Oil extract: a novel green inhibitor for mild steel corrosion in 1 M HCl solution. Int J Electrochem Sci 7:1303–1318
Znini M, Bouklah M, Majidi L, Kharchouf S, Aouniti A, Bouyanzer A, Hammouti B, Costa J, Al-Deyab SS (2011) Chemical composition and inhibitory effect of Mentha spicata essential oil on the corrosion of steel in molar hydrochloric acid. Int J Electrochem Sci 6:691–704
Boumhara K, Tabyaoui M, Jama C, Bentiss F (2015) Artemisia mesatlantica essential oil as green inhibitor for carbon steel corrosion in 1 M HCl solution: electrochemical and XPS investigations. J Ind Eng Chem 29:146–155
Archana S, Anurag S, Deepti S, Praveen J (2012) Corrosion inhibition and adsorption behavior of clove oil on iron in acidic medium. E J Chem 9:2044–2051
Abdulwahab M, Fayomi OSI, Popoola API, Asuke F, Umoru LE (2014) Effect of Avogadro natural oil on the corrosion inhibition of mild steel in hydrochloric acid solution. Res Chem Intermed 40:1115–1123
Afia L, Salghi R, Bammou L, El Bazzi, Hammouti B, Bazzi L, Bouyanzer A (2014) Anti-corrosive properties of Argan oil on C38 steel in molar HCl solution. J Saudi Chem Soc 18:19–25
Halambek J, Berkovic K, Vorkapic-Furac KJ (2010) The influence of L. angustifolia L. oil on corrosion of Al-3 Mg alloys. Corros Sci 52:3978–3983
Znini M, Paolini J, Majidi L, Desjobert J-M, Costa J, Lahhit N, Bouyanzer A (2012) Evaluation of the inhibitive effect of essential oil of L. multifida L. on the corrosion behavior of C38 steel in 0.5 M H2SO4 medium. Res Chem Intermed 38:669–683
Boudalia M, Guenbour A, Bellaouchou A, Laqhaili A, Mousaddak M, Hakiki A, Hammouti B, Ebenso EE (2013) Corrosion inhibition of organic oil extract of leaves of L. stoekas on stainless steel in concentrated phosphoric acid solution. Int J Electrochem Sci 8:7414–7424
Zerga B, Sfaira M, Rais Z, Ebn Touhami M, Taleb M, Hammouti B, Imelouane B, Elbachiri A (2009) Lavender oil as an ecofriendly inhibitor for mild steel in 1 M HCl. Matériaux Techn 97:297–305
Saadi A, Brada M, Kouidri M, Dekkiche H, Attar F (2016) Chemical composition and content of essential oil of Lavandula multifidia from Algeria. Chem Nat Compd 52:162–164
Merah S, Larabi L, Benali O, Harek Y (2008) Synergistic effect of methyl red dye and potassium iodide on inhibition of corrosion of carbon steel in 0.5 M H2SO4. Pigm Resin Technol 37:291–298
Lebrini M, Traisnel M, Lagrenée M, Mernari B, Bentiss F (2008) Inhibitive properties, adsorption and a theoretical study of 3,5-bis(n-pyridyl)-4-amino-1,2,4-triazoles as corrosion inhibitors for mild steel in perchloric acid. Corros Sci 50:473–479
Nabel AN, Emad AB, Zakaria K, Mohamed A (2015) Environmentally friendly nonionic surfactants derived from Jatropha oil fatty acids as inhibitors for carbon steel corrosion in acidic medium. J Surfactants Deterg 18:1011–1024
Bommersbach P, Alemany-Dumont C, Millet JP, Normand B (2006) Hydrodynamic effect on the behaviour of a corrosion inhibitor film: characterization by electrochemical impedance spectroscopy. Electrochim Acta 51:4011–4018
Benali O, Larabi L, Merah S, Harek Y (2011) Influence of the methylene blue dye (MBD) on the corrosion inhibition of mild steel in 0.5 M sulphuric acid, part I: weight loss and electrochemical studies. J Mater Environ Sci 2:39–48
Larabi L, Harek Y, Benali O, Ghalem S (2005) Hydrazide derivatives as corrosion inhibitors for mild steel in 1 M HCl. Prog Org Coat 54:256–262
Khaled KF, Amin MA (2009) Corrosion monitoring of mild steel in sulphuric acid solutions in presence of some thiazole derivatives-molecular dynamics, chemical and electrochemical studies. Corros Sci 51:1964–1975
Kissi M, Bouklah M, Hammouti B, Benkaddour M (2006) Establishment of equivalent circuits from electrochemical impedance spectroscopy study of corrosion inhibition of steel by pyrazine in sulphuric acidic solution. Appl Surf Sci 252:4190–4197
Merah S, Larabi L, Abderrahim O, Harek Y (2017) Study of corrosion inhibition of C38 steel in 1 M HCl solution by polyethyleneiminemethylene phosphonic acid. Int J Ind Chem 8:263–272
Malki AL, Kertit S, Bellaouchou A, Guenbour A, Benbachir A, Hammouti B (2008) Phosphate of aluminum as corrosion inhibitor for steel in H3PO4. Port Electroch Acta 26:339–347
Chaubey N, Yadav DK, Singh VK, Quraishi MA (2017) A comparative study of leaves extracts for corrosion inhibition effect on aluminium alloy in alkaline medium. Ain Shams Eng J 8:673–682
Behpour M, Ghoreishi SM, Khayatkashani M, Soltani N (2011) The effect of two oleo-gum resin exudate from Ferula assa-foetida and Dorema ammoniacum on mild steel corrosion in acidic media. Corros Sci 53:2489–2501
Li XH, Deng SD, Fu H (2010) Adsorption and inhibition effect of vanillin on cold rolled steel in 3.0 M H3PO4. Prog Org Coat 67:420–426
Bouklah M, Hammouti B, Lagrenee M, Bentiss F (2006) Thermodynamic properties of 2,5-bis(4-methoxyphenyl)-1,3,4-oxadiazole as a corrosion inhibitor for mild steel in normal sulfuric acid medium. Corros Sci 48:2831–2842
Mernari B, Elkadi L, Kertit S (2001) 2, 5-bis(2-Thienyl)-1, 3, 4-oxadiazole as corrosion inhibitor of mild steel in acidic media. Bull Electrochem 17:115–120
Szauer T, Brandt A (1981) On the role of fatty acid in adsorption and corrosion inhibition of iron by amine-fatty acid salts in acidic solution. Electrochim Acta 26:1219–1224
Foroulis ZA (1990). In: proceeding of 7th European corrosion inhibitors, Ferrara. p 144
Acknowledgements
The authors extend their appreciation to all researchers who have contributed to the realization of this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Belarbi, N., Dergal, F., Chikhi, I. et al. Study of anti-corrosion activity of Algerian L. stoechas oil on C38 carbon steel in 1 M HCl medium. Int J Ind Chem 9, 115–125 (2018). https://doi.org/10.1007/s40090-018-0143-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40090-018-0143-6