Abstract
A new class of corrosion inhibitors, namely, polyethyleneiminemethylene phosphonic acid (PEIMPA), was synthesized and its inhibiting action on the corrosion of C38 steel in 1 M HCl at 30 °C was investigated by various corrosion monitoring techniques such as weight loss measurements, potentiodynamic polarization, linear polarization resistance (Rp), and surface analysis (SEM and EDX) which are used to characterize the steel surface. Weight loss measurements revealed that the presence of PEIMPA increases the inhibition efficiency by decreasing the corrosion rate. Tafel polarization study showed that the inhibitor acts as a mixed-type inhibitor. Adsorption of PEIMPA on the carbon steel surface was found to obey the Langmuir isotherm. Some thermodynamic functions of dissolution and adsorption processes were also determined and discussed. The SEM results showed the formation of protective film on the mild steel surface in the presence of PEIMPA. The results obtained from different tested techniques were in good agreement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Study of organic corrosion inhibitor is an attractive field of research due to its usefulness in various industries. Acid is widely used in various industries for the pickling of ferrous alloys and steels. Because of the aggressive nature of the acid medium, the inhibitors are commonly used to reduce acid attack on the substrate metal. Most of the reported corrosion inhibitors are organic compounds containing O, N, S, and P [1,2,3,4,5,6,7,8,9,10,11,12,13,14] in their structures. The phosphoric functions are considered to be the most effective chemical group against corrosion process [15]. The use of organic phosphonic acids to protect carbon steel against corrosion has been the subject of various works [16,17,18,19,20,21,22,23,24,25,26]. Aminomethyl-phosphonic acids are excellent sequestering agents for electroplating, chemical plating, degreasing, and cleaning. It was shown that piperidin-1-yl-phosphonic acids (PPA) and (4-phosphono-piperazin-1-yl) phosphonic acid (PPPA) are used to reduce the corrosion of iron in a NaCl medium, even if PPPA is more efficient than PPA [27]. In the present investigation, the influence of polyethyleneiminemethylene phosphonic acid (PEIMPA) as a corrosion inhibitor of carbon steel in 1 M HCl has been systematically studied by weight loss measurements, potentiodynamic polarization studies, and surface analysis (SEM, EDX). Results are reported and discussed.
Experimental
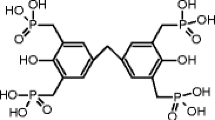
Polyethyleneiminemethylene phosphonic acid polymer was synthesized (see Scheme 1) from commercially available Lupasol P (polyethylenimine) according to the Moedrizer–Irani reaction [28]. The synthesis was performed in distilled water under microwave irradiation. In a quartz reactor, a mixture of polyethylenimine (Lupasol P, 80 mmol, 3.44 g), phosphorous acid (80 mmol, 6.68 g), and hydrochloric acid–water (1:1) solution (12 mL) was vigorously stirred and then irradiated (150 W) in a glass cylinder reactor for 1 min. A formaldehyde aqueous solution (160 mmol) was added and irradiated for 8 min.
Then, the precipitation was washed with distilled water to remove unreacted reagents. Finally, phosphonic-modified Lupasol P was washed three times with distilled water and ethanol. After drying, the solid was further pulverized to give a brown powder.
The structure and purity were identified and characterized by elemental microanalysis (Table 1) and 1H, 13C, and 31P NMR spectroscopy. The spectra showed the expected signals due to the polyethylenimine skeleton and methylene phosphonic units as matched to the proposed structure (Scheme 1).
NMR spectral data: 1H NMR d (ppm): 4.92 (N–CH2); 2.33 (CH2–P); 1.6 NH. 13C NMR d (ppm): 82.16 (N–CH2), 52.1 (CH2–P). 31P NMR d (ppm): 3.91. The presence of phosphonic acid was confirmed by FTIR measurement: the polymer displays characteristic bonds for P–O–C at 1050 cm−1, P–OH at 2372, and 2338 cm−1 189 and P = O at 1172 cm−1.
Elemental microanalysis suggests the structure made of fragment of the phosphonic acid polymer, corresponding after calculation to x = 5 and y = 9 (Scheme 1).
A 1 M HCl solution was prepared from an analytical reagent grade of HCl 37% and double-distilled water and was used as corrosion media in the studies. Note that the solubility of polyethyleneiminemethylene phosphonic acid is very high in this medium.
For the weight loss measurements, the experiments were carried out in the solution of 1 M HCl (uninhibited and inhibited) on carbon steel containing 0.30–0.35% C, 0.15–0.35% Si, 0.035% S, 0.5–1.0% Mn, and 0.035% P. Sheets with dimensions 20 mm × 10 mm × 2 mm were used. They were polished successively with different grades of emery paper up 1200 grade. Each run was carried out in a glass vessel containing 100 ml test solution. A clean weight mild steel sample was completely immersed at an inclined position in the vessel. After 4 h of immersion in 1 M HCl with and without the addition of inhibitor at different concentrations, the specimen was withdrawn, rinsed with double-distilled water, washed with acetone, dried, and weighed. The weight loss was used to calculate the corrosion rate in milligrams per square centimeter per hour.
Electrochemical experiments were carried out in a glass cell (CEC/TH Radiometer) with a capacity of 500 ml. A platinum electrode and a saturated calomel electrode (SCE) were used as a counter electrode and a reference electrode. The working electrode was in the form of a disc cut from mild steel under investigation and was embedded in a Teflon rod with an exposed area of 0.5 cm2. Potentiodynamic polarizations were conducted in an electrochemical measurement system (VoltaLab 21) which comprises a PGP201 potentiostat, a personal computer, and VoltaMaster4 software. The polarization resistance measurements were performed by applying a controlled potential scan over a small range typically ±15 mV with respect to Ecorr with a scanning rate of 0.5 mV s−1. The resulting current is linearly plotted vs. potential, the slope of this plot at Ecorr being the polarization resistance (Rp). Corrosion current densities were determined by extrapolating the cathodic Tafel regions from the potentiodynamic polarization curves to the corrosion potential. The potentiodynamic current–potential curves were recorded by changing the electrode potential automatically from −700 to −300 mV with the same scanning rate (0.5 mV s−1) under static on the same electrode without any surface treatment. All experiments were carried out in freshly prepared solution at constant temperatures.
Inhibition efficiencies P % were calculated as follows:
Weight loss measurement:
where \( w \) and \( w^{{\prime }} \) are the corrosion rate of steel due to the dissolution in 1 M HCl in the absence and the presence of definite concentrations of inhibitor, respectively.
Linear polarization measurement:
where \( R_{p} \) and \( R_{\text{p}}^{{\prime }} \) are the values of linear polarization in the absence and presence of the inhibitor, respectively.
Polarization measurement:
where \( i_{\text{corr}} \) and \( i_{\text{corr}}^{{\prime }} \) are the corrosion current densities in the absence and the presence of the inhibitor, respectively.
For all methods, the tests were performed in non-de-aerated solutions under unstirred conditions.
The surface morphology of the samples before and after adding PEIMPA inhibitor in the medium 1 M HCl after 1 day of immersion was observed by scanning electron microscope (SEM) Quanta 200 FEG coupled with EDX analysis.
Results and discussion
Weight loss measurements
The gravimetric measurements of mild steel in 1 M HCl in the absence and presence of various concentrations of PEIMPA investigated were determined after 4 h of immersion at 30 °C.
Table 2 gives values of the corrosion rates and percentage inhibition efficiency calculated from the weight loss measurements for different concentrations of PEIMPA.
Inspection of this table shows that the inhibition efficiency increases with increasing inhibitor concentration. The optimum concentration required to achieve this efficiency is found to be 500 ppm. The inhibition of corrosion of carbon steel by the investigated inhibitor can be explained in terms of adsorption on the metal surface. It is generally assumed that the adsorption of the inhibitor at the metal/solution interface is the first step in the mechanism of inhibition in aggressive media.
This compound can be adsorbed on the metal surface by the interaction between lone pair of electrons of hetero atoms and the metal surface. This process is facilitated by the presence of vacant orbitals d of low energy in iron atom, as observed in the transition group metals. Moreover, the formation of positively charged protonated species in acidic solutions facilitates the adsorption of the compound on the metal surface through electrostatic interactions between the organic molecules and the metal surface [29].
Polarization measurements
Figure 1 shows the polarization curves of mild steel in 1 M HCl, blank solution, and in the presence of different concentrations (100–500 ppm) of PEIMPA. With the increase of PEIMPA concentrations, both anodic and cathodic currents were inhibited. This result shows that the addition of PEIMPA inhibitor reduces anodic dissolution and also retards the hydrogen evolution reaction. We note that the corrosion potential varies slightly after the addition of the inhibitor at different concentrations.
Table 3 shows that an increase in inhibitor concentration is resulted in increased inhibition efficiency. It is evident from the results that the I corr values decrease considerably in the presence of inhibitor and that the maximum decrease in I corr coincides with the optimum concentration of inhibitor. Linear polarization technique was performed in 1 M HCl with various concentrations of PEIMPA. The corresponding polarization resistance (R p) values of carbon steel in the absence and in the presence of different inhibitor concentrations are also given in Table 3. It is apparent that R p increases with increasing inhibitor concentration. The inhibition percentage (P %) calculated from R p values is also presented in Table 3. We remark that P % increases with increasing concentration of inhibitor and attains 87% at 500 ppm. The inhibition efficiencies of PEIMPA obtained by potentiodynamic polarization and by polarization resistance methods are in good agreement, particularly, at high concentrations.
For anodic polarization, it can be seen from Fig. 1 that, in the presence of PEIMPA at all concentrations, two linear portions were observed. When the anodic potentials increases, the anodic current increases at a slope of b a1 in the low polarization potential region. After passing a certain potential E u, the anodic current increases rapidly and dissolves at a slope of b a2 in the high polarization region. This behavior was already documented for iron in acid solutions [30,31,32,33]. The rapid increase of anodic current after E u may be due to the desorption of PEIMPA molecules adsorbed on the electrode. This means that the inhibition mode of PEIMPA depends on electrode potential. In this case, the observed inhibition phenomenon is generally described as corrosion inhibition of the interface associated with the formation of a bidimensional layer of adsorbed inhibitor species at the electrode surface [34]. Note that the potential E u is also denoted E 1 in Bartos and Hackerman’s paper [30]. Figure 1 shows also that, at potentials higher than E corr, PEIMPA affects the anodic reaction. This result indicates that PEIMPA exhibits both anodic and cathodic inhibition effects.
Adsorption isotherm
The adsorption of the organic compounds can be described by two main types of interaction: physical adsorption and chemisorption that are influenced by the charge nature of the metal, the type of the electrolyte, and the chemical structure of the inhibitor.
The adsorption isotherm can give information on the metal–inhibitor interaction. The adsorption isotherm can be derived from the curve surface coverage against inhibitor concentration. Surface coverage \( \theta \) was estimated as in Eq. 5. The \( \theta \) values for different inhibitor concentrations are tested by fitting to various isotherms. So far, the best fit was obtained with the Langmuir isotherm. According to this isotherm, \( \theta \) is related to concentration inhibitor C via
where C is the concentration of inhibitor, K is the adsorptive equilibrium constant, and θ is the surface coverage and calculated by the following equation [6, 35, 36]:
where \( I_{\text{corr}}^{'} \) is the corrosion current density in uninhibited acid and \( I_{\text{corr}} \) is the corrosion current density in inhibited acid.
Plotting C/θ vs. C yields a straight line, as shown in Fig. 2. The linear correlation coefficient (r) is almost equal to 1 (r = 0.9999) and the slight deviation of the slope (1.14) from the unity is attributable to molecular interactions in the adsorbed layer which corresponds to the observed physical adsorption mechanism [37]. The adsorptive equilibrium constant (K) value is 6.22 × 105 L mol−1. The free energy of adsorption ΔG°ads of the inhibitor on mild steel surface can be determined using the following relation:
where R is the gas constant (8.314 J K−1 mol−1), T is the absolute temperature (K), and the value 55.5 is the concentration of water in solution expressed in M. The ∆G° ads value calculated is −37.90 kJ mol−1. The negative values of ∆G° ads indicate that the adsorption of inhibitor molecule onto steel surface is a spontaneous process. In general, it is well known that the values of −ΔG°ads of the order of −20 kJ mol−1 or lower indicate a physisorption; those of order of −40 kJ mol−1 or higher involve charge sharing or a transfer from the inhibitor molecules to the metal surface to form a coordinate type of bond (chemisorption). In the present study, the value of ΔG°ads is about between −20 and −40 kJ mol−1, probably mean that the adsorption mechanism of the PEIMPA on steel in 1 M HCl solution is both physisorption and chemisorption.
Noticeably, it is generally accepted that physical adsorption is the preceding stage of chemisorption of inhibitors on metal surface [38]. It was reported that in this domaine, the surface charge of steel at E corr in HCl solution is expected to be positive. Thus, the anions are first adsorbed on the steel surface creating an excess negative charge, which, in turn, facilitates physical adsorption of the inhibitor cations [39]. Accordingly, the Cl− and phosphonate ions adsorb and the surface becomes negatively charged. Due to the electrostatic attraction, the protonated PEIMPA molecules are adsorbed on carbon steel surface (physisorption).
Along with electrostatic force of attraction, inhibitor also adsorbs on the carbon steel surface through chemical adsorption. The adsorption of free molecules could take place via interaction of the unshared pairs of electrons of nitrogen and oxygen atoms of the –PO(OH)2 group and the vacant d-orbitals of iron atoms.
Therefore, inhibition takes place through both physisorption and chemisorption. However, chemisorption has no substantial contribution [12]. Indeed, the PEIMPA molecules are easily protonated to form ionic forms in acid solution. It is logical to assume that in this case, the electrostatic cation adsorption is mainly responsible for the protective properties of this compound.
Effect of temperature
To investigate the mechanism of inhibition and to determine the activation energy of the corrosion process, polarization curves of steel in 1 M HCl were determined at various temperatures (303–333 K) in the absence and presence of 500 ppm of PEIMPA. Representative Tafel polarization curves for C38 steel electrode in 1 M HCl without and with 500 ppm at different temperatures are shown in Fig. 3a, b. Similar polarization curves were obtained in the case of the other concentrations of PEIMPA (not given). The analysis of these figures reveals that raising the temperature increases both anodic and cathodic current densities, and consequently, the corrosion rate of C38 steel increases. The corresponding data are given in Table 4. In the studied temperature range (303–333 K), the corrosion current density increases with increasing temperature both in uninhibited and inhibited solutions and the values of the inhibition efficiency of PEIMPA decrease with the increase of temperature.
Figure 4 and 5 present the Arrhenius plots of the natural logarithm of the current density vs. 1/T, for 1 M solution of hydrochloride acid, without and with the addition of PEIMPA and ln(i corr/T) with reciprocal of the absolute temperature, respectively. Straight lines with coefficients of correlation (c.c.) high to 0.99 are obtained for the supporting electrolyte and PEIMPA.
The values of the slopes of these straight lines permit the calculation of the Arrhenius activation energy, E a, according to
where R is the universal gas constant and A is the Arrhenius factor.
In addition, from transition-state plot according to the following equation:
where ∆H°a is the enthalpy of activation and B is a constant.
The E a and ΔH°a values were determined from the slopes of these plots. The calculated values of E a and ΔH a in the absence and the presence of PEIMPA at 500 ppm are given in Table 5. Inspection of these data reveals that the increase in E a in the presence of the inhibitor may be interpreted as physical adsorption. Indeed, a higher energy barrier for the corrosion process in the inhibited solution is associated with physical adsorption or weak chemical bonding between the inhibitor species and the steel surface [22, 40]. Szauer and Brand explained that the increase in activation energy can be attributed to an appreciable decrease in the adsorption of the inhibitor on the carbon steel surface with the increase in temperature. A corresponding increase in the corrosion rate occurs because of the greater area of metal that is consequently exposed to the acid environment [41].
The enthalpy of activation values is found to be positive in the absence and presence of inhibitor and reflects the endothermic mild steel dissolution process. It is evident from Table 5 that the value of ΔH° a increased in the presence of PEIMPA than the uninhibited solution indicating protection efficiency. This suggested the slow dissolution and hence lower corrosion rate of mild steel [2, 7]. This result permits verifying the known thermodynamic equation between E a and ΔH° a [42]:
Surface examination by SEM/EDX
Figure 6 shows the scanning electron micrographs of C38 after immersion for 24 h in 1 M HCl solution. The specimen surface in Fig. 6 appears to be roughened extensively by the corrosive environment and the porous layer of corrosion product is present. The EDX spectra show the characteristics peaks of some of the elements constituting of the steel sample after 24 h immersion in 1 M HCl without inhibitor, which reveals the presence of oxygen and iron, suggesting, therefore, the presence of iron oxide/hydroxide.
When PEIMPA was added into the corrosion test solution (Fig. 7), a smooth surface was noticed traducing a good protection effect of the corrosion inhibitor by a formation of a thick and compact film. This may be intercepted by the adsorption of these inhibitors on the electrode surface.
In the presence of PEIMPA inhibitor, the EDX spectra show additional lines of nitrogen and phosphorus, due to the adsorbed layer of inhibitor that covered the electrode surface. In addition, the Fe peaks are decreasing in relation to the uninhibited steel surface sample. This diminution of the Fe lines occurs because of the overlying inhibitor film.
These results confirm those from weight loss and polarization measurements, which suggest that a protective film was formed over the metal surface and hence retarded both anodic and cathodic reactions.
Conclusion
On the basis of this study, the following conclusions can be drawn:
-
PEIMPA shows a very good activity of preventing corrosion of carbon steel in 1 M HCl.
-
Inhibition efficiency of PEIMPA varies directly with the concentration and inversely with the temperature.
-
Potentiodynamic polarization experiments reveal that PEIMPA acts as mixed-type inhibitor.
-
The adsorption of PEIMPA on the metal surface follows Langmuir isotherm.
-
The corrosion inhibition is probably due to the adsorption of PEIMPA on the metal surface and blocking its active sites by the phenomenon of physical and chemical adsorptions.
-
The weight loss, linear polarization, and polarization curves are in good agreement.
-
SEM and EDX techniques reveal that the inhibitor molecules form a good protective film on the steel surface and confirm the result obtained by gravimetric and polarization methods.
References
Larabi L, Harek Y, Benali O, Ghalem S (2005) Hydrazide derivatives as corrosion inhibitors for mild steel in 1 M HCl. Prog Org Coat 54:256–262
Benali O, Larabi L, Tabti B, Harek Y (2005) Influence of 1-methyl 2-mercapto imidazole on corrosion inhibition of carbon steel in 0.5 M H2SO4. Anti Corros Method Mater 52:280–285
Benali O, Larabi L, Mekelleche SMB, Harek Y (2006) Influence of substitution of phenyl group by naphthyl in a diphenylthiourea molecule on corrosion inhibition of cold-rolled steel in 0.5 M H2SO4. J Mat Sci 41:7064–7073
Larabi L, Benali O, Harek Y (2007) Corrosion inhibition of cold rolled steel in 1 M HClO4 solutions by N-naphtyl N′-phenylthiourea. Mat Lett 61:3287–3291
Benali O, Larabi L, Traisnel M, Gengembre L, Harek Y (2007) Electrochemical, theoretical and XPS studies of 2-mercapto-1-methylimidazole adsorption on carbon steel in 1 M HClO4. Appl Surf Sci 253:6130–6139
Benali O, Larabi L, Merah S, Harek Y (2011) Influence of the methylene blue dye (MBD) on the corrosion inhibition of mild steel in 0.5 M sulphuric acid, part I: weight loss and electrochemical studies. J Mater Environ Sci 2:39–48
Sabirneeza AAF, Subhashini S (2014) Poly(vinyl alcohol–proline) as corrosion inhibitor for mild steel in 1 M hydrochloric acid. Int J Ind Chem 5:111–120
Touhami F, Aouniti A, Kertit S, Abed Y, Hammouti B, Ramdani A, El-Kacemi K (2009) Corrosion inhibition of armco iron in 1 M HCl media by new bipyrazolic derivatives. Corros Sci 42:929–940
Bouklah M, Hammouti B, Aouniti A, Benhadda T (2004) Thiophene derivatives as effective inhibitors for the corrosion of steel in 0.5 M H2SO4. Prog Org Coat 47:225–228
Bouklah M, Hammouti B, Lagrenée M, Bentiss F (2006) Thermodynamic properties of 2,5-bis(4-methoxyphenyl)-1,3,4-oxadiazole as a corrosion inhibitor for mild steel in normal sulfuric acid medium. Corros Sci 48:2831–2842
Kertit S, Essouffi H, Hammouti B, Benkaddour B (1998) 1-phenyl-5-mercapto-1,2,3,4-tétrazole (PMT): un nouvel inhibiteur de corrosion de l’alliage Cu-Zn efficace à très faible concentration. J Chim Phys 95:2072–2082
Labjar N, Lebrini M, Bentiss F, Chihib NE, Hajjaji SE, Jama C (2010) Corrosion inhibition of carbon steel and antibacterial properties of aminotris-(methylenephosphonic) acid. Mater Chem Phys 119:330–336
Amar H, Benzakour J, Derja A, Villemin D, Moreau B, Braisaz T (2006) Piperidin-1-yl-phosphonic acid and (4-phosphono-piperazin-1-yl) phosphonic acid: a new class of iron corrosion inhibitors in sodium chloride 3% media. Appl Surf Sci 252:6162–6172
Amar H, Benzakour J, Derja A, Villemin D, Moreau BJ (2003) A corrosion inhibition study of iron by phosphonic acids in sodium chloride solution. J Electroanal Chem 558:131–139
Truc TA, Pebere N, Hang TTX, Hervaud Y, Boutevin B (2002) Study of the synergistic effect observed for the corrosion protection of a carbon steel by an association of phosphates. Corros Sci 44:2055–2071
Andijani I, Turgoose S (2003) Studies on corrosion of carbon steel in deaerated saline solutions in presence of scale inhibitor. Desalination 123:223–231
Rajendran S, Reenkala SM, Anthony N, Ramaraj R (2002) Synergistic corrosion inhibition by the sodium dodecylsulphate–Zn2+ system. Corros Sci 44:2243–2252
To XH, Pebere N, Pelaprat N, Boutevin B, Hervaud Y (1997) A corrosion-protective film formed on a carbon steel by an organic phosphonate. Corros Sci 39:1925–1934
Rajendran S, Apparao BV, Palaniswamy N (1999) Synergistic effect of 1-hydroxyethane-1, 1-diphosphonic acid and Zn2+ on the inhibition of corrosion of mild steel in neutral aqueous environment. Anti Corros Method Mater 46:23–28
Pech MA, Chi-Canul LP (1999) Investigation of the Inhibitive Effect of N-phosphono-methyl-glycine on the corrosion of carbon steel in neutral solutions by electrochemical techniques. Corrosion 55:948–956
Nakayama N (2000) Inhibitory effects of nitrilotris(methylenephosphonic acid) on cathodic reactions of steels in saturated Ca(OH)2 solutions. Corros Sci 42:1897–1920
Gunasekharan G, Natarajan R, Palaniswamy N (2001) The role of tartrate ions in the phosphonate based inhibitor system. Corros Sci 43:1615–1626
Rajendran S, Apparao BV, Palaniswamy N, Periasamy V, Karthikeyan G (2001) Corrosion inhibition by strainless complexes. Corros Sci 43:1345–1354
Bouklah M, Krim O, Messali M, Hammouti B, Elidrissi A (2011) A pyrrolidine phosphonate derivative as corrosion inhibitor for steel in H2SO4 solution. Warad I Der Pharma Chim 3:283–293
Dkhireche N, Abdelhadi R, Ebn Touhami M, Oudda H, Touir R, Elbakri M, Sfaira M, Hammouti B, Senhaji O, Taouil R (2012) Elucidation of dimethyldodecylphosphonate and CTAB synergism on corrosion and scale inhibition of mild steel in simulated cooling water system. Int J Electrochem Sci 7:5314–5330
Kharbach Y, Haoudi A, Skalli MK, Kandri Rodi Y, Aouniti A, Hammouti B, Senhaji O, Zarrouk A (2015) The role of new phosphonate derivatives on the corrosion inhibition of mild steel in 1 M H2SO4 media. J Mater Environ Sci 6:2906–2916
Villemin D, Didi MA (2015) Aminomethylenephosphonic acids syntheses and applications (A Review). Orient J Chem 31:01–12
Ferrah N, Abderrahim O, Didi MA, Villemin D (2011) Removal of copper ions from aqueous solutions by a new sorbent: polyethyleneiminemethylene phosphonic acid. Desalination 269:17–24
Merah S, Larabi L, Benali O, Harek Y (2008) Synergistic effect of methyl red dye and potassium iodide on inhibition of corrosion of carbon steel in 0.5 M H2SO4. Pigm Resin Technol 37:291–298
Bartos M, Hackerman N (1992) A Study of inhibition action of propargyl alcohol during anodic dissolution of iron in hydrochloric acid. J Electrochem Soc 139:3428–3433
Kuo HC, Nobe KJ (1978) Electrodissolution kinetics of iron in chloride solutionsVI. Concentrated acidic solutions. J Electrochem Soc 125:853–860
Mac Farlane DR, Smedley SI (1986) The dissolution mechanism of iron in chloride solutions. J Electrochem Soc 133:2240–2244
Feng Y, Siow KS, Teo WK, Hseieh AK (1999) The synergistic effects of propargyl alcohol and potassium iodide on the inhibition of mild steel in 0.5 M sulfuric acid solution. Corros Sci 41:829–852
Lorentz WJ, Mansfeld F (1986) Interface and interphase corrosion inhibition. Corros Sci 31:467–476
Tsuru T, Haruyama S, Gijutsu B (1978) Corrosion inhibition of iron by amphoteric surfactants in 2 M HCl. J Jpn Soc Corros Eng 27:573–581
Xianghong L, Shuduan D, Hui F (2010) Blue tetrazolium as a novel corrosion inhibitor for cold rolled steel in hydrochloric acid solution. Corros Sci 52:2786–2792
Malki Alaoui L, Kertit S, Bellaouchou A, Guenbour A, Benbachir A, Hammouti B (2008) Phosphate of aluminum as corrosion inhibitor for steel in H3PO4. Portug Electroch Acta 26:339–347
Wang FP, Kang WL, Jin HM (2008) Corrosion electrochemistry mechanism, methods and applications. Chemical Industrial Engineering Press, Beijing, p 242
Popova A, Sokolova E, Raicheva S, Christov M (2003) AC and DC study of the temperature effect on mild steel corrosion in acid media in the presence of benzimidazole derivatives. Corros Sci 45:33–58
Bentiss F, Lebrini M, Lagrenee M (2005) Thermodynamic characterization of metal dissolution and inhibitor adsorption processes in mild steel/2,5-bis(n-thienyl)-1,3,4-thiadiazoles/hydrochloric acid system. Corros Sci 47:2915–2931
Szauer T, Brandt A (1981) On the role of fatty acid in adsorption and corrosion inhibition of iron by amine-fatty acid salts in acidic solution. Electrochim Acta 26:1219–1224
Laidler KJ (1963) Reaction kinetics, vol 1, 1st edn. Pergamon Press, New York
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Salah, M., Lahcène, L., Omar, A. et al. Study of corrosion inhibition of C38 steel in 1 M HCl solution by polyethyleneiminemethylene phosphonic acid. Int J Ind Chem 8, 263–272 (2017). https://doi.org/10.1007/s40090-017-0123-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40090-017-0123-2