Abstract
Electrically conductive polypyrrole–zirconium(IV) phosphate (PPy–ZrP) cation exchange nanocomposites have been synthesized for the first time by in situ chemical oxidative polymerization of pyrrole in the presence of zirconium(IV) phosphate (ZrP). Fourier Transform Infra-red spectroscopy (FTIR), field emission scanning electron microscopy, transmission electron microscopy, X–ray diffraction, thermogravimetric analysis, differential thermal analysis, derivative thermogravimetry and elemental analysis were used to characterize PPy–ZrP cation exchange nanocomposite. The composite showed good ion-exchange capacity (1.60 meq g−1), DC electrical conductivity (0.33 S cm−1) and isothermal stability in terms of DC electrical conductivity retention under ambient condition up to 100 °C. PPy–ZrP cation exchangenanocomposite-based sensor was fabricated for the detection of ammonia vapours of aqueous ammonia. The resistivity of the nanocomposites increases on exposure to high-concentration ammonia vapours at room temperature (25 °C). The rate of reaction for ammonia vapour-sensing on PPy–ZrP was observed as second order.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years electrically conducting polymers have received much attention for use as advanced materials due to their good physical attributes [1–3]. Among the various conducting polymers such as polypyrrole, polythiophene, polyaniline, etc., polypyrrole is an especially promising electrically conducting polymer for commercial applications due to its high conductivity, good environmental stability and ease in synthesis. In addition, polypyrrole is one of the most familiar conducting polymers that show many advantages in recombining millimicron particles to give nanocomposites [4]. Nanocomposite show new properties due to synergism between the constituents [5–10] and more available surface. Because of the new properties, nanocomposites may find applications in various fields such as device fabrication [11], photo catalysis [12], solar cells [13], fuel cell [14], biomedical and sensing application [15].
Various polypyrrole-based composites such as palladium–polypyrrole nanocomposite, polypyrrole-Au nanocomposite, polypyrrole-TiO2 nanocomposite, polypyrrole-manganese oxide composite, etc. are synthesized and used for ammonia sensing [16–18], DNA bio sensing, in super capacitor electrode, etc. [17]. However, conducting polymer-based ion exchangers with polyvalent sites have been poorly reported in the field of gas sensing [6–8]. Conducting ion-exchange materials having millimicron particles are considered an advanced class of materials because of its excellent ion-exchange behaviour and their analytical as well as electro-analytical applications [19–21].
Thus, in this work, we have synthesized a new electrically conductive ion-exchange nanocomposite PPy–ZrP by in situ oxidative chemical polymerization technique and used it as new sensing material for ammonia vapour at room temperature. To the best of our knowledge, this is the first attempt to synthesize a new electrically conductive PPy–ZrP cation exchange nanocomposite by using in situ oxidative chemical polymerization technique and applied for ammonia vapour sensing characteristics.
Experimental
Chemical, reagents and instruments
The following reagents and instruments were used:
The Pyrrole monomer (98%) from Spectrochem (India Ltd.), anhydrous Iron(III)-chloride (FeCl3), methanol HPLC grade Ortho phosphoric acid (H3PO4) and Zirconium oxychloride were used as received from Qualigens (India Ltd.).All other reagents and chemicals were of analytical grade.
Ultrasonic vibrations (SC-I, Chengdu Jiuzhou Ultrasonic Technology Co.), Fourier Transform Infra-red spectroscopy (FTIR) (Perkin Elmer 1725 instrument), Transmission electron microscopy (TEM) (JEOL TEM, JEM 2100F), field emission scanning electron microscopy (FE-SEM) and energy-dispersive analyzer unit (EDAX) (LEO 435-VF), X-ray diffraction (XRD) (PHILIPS PW1710 diffractometer), Thermal analysis (TGA, DTA and DTG) (thermal analyzer-V2.2A DuPont 9900).
Synthesis
Zirconium(IV) phosphate
Preparation of ZrP was carried out by taking different ratios of zirconium oxychloride solution and aqueous solution of orthophosphoric acid (prepared in demineralized water) under varying conditions given in Table 1. The reaction mixture was thoroughly stirred with a magnetic stirrer at room temperature (25 °C), the solution containing precipitate was stirred for 1 h and was refluxed at 75–80 °C for 24 h. The resulting precipitate was decanted and washed with demineralized water (DMW), filtered by suction and dried at 50 ± 2 °C for 24 h. The excess of acid was removed by repeated washing with DMW. Finally, the material was dried in an oven at 50 ± 2 °C for 4 h and ground by pastel mortar to obtain a fine powder of ZrP.
Polypyrrole–zirconium(IV) phosphate nanocomposite
PPy–ZrP nanocomposites were prepared by in situ chemical oxidative polymerization [5–8] of pyrrole in the presence of ZrP particles. A schematic representation of the formation of PPy–ZrP nanocomposite is shown in Scheme 1. A certain amount of ZrP (dried at 50 °C for 2 h before use) was dispersed in 100 ml of double-distilled water (DDW) under ultrasonic vibrations (SC-I, Chengdu Jiuzhou Ultrasonic Technology Co.) at room temperature for 1 h. This ZrP dispersed solution was then diverted into a 500-mL single-necked, round-bottom flask equipped with a magnetic Teflon-coated stirrer, and a certain amount of pyrrole monomer was added. The mixture was stirred for 30 min for the adsorption of pyrrole on the surface of ZrP particles. 2 g Ferric chloride in 100 ml of DDW was added to the dispersion. This reaction mixture was stirred for an additional 24 h under the same condition. The resultant PPy–ZrP nanocomposite powder was filtered using a Buchner funnel and then washed with DMW to remove unreacted oxidant. It was further washed thoroughly with methanol to remove any unreacted polymer. The obtained powders were dried completely at 50 °C for further analysis. Pure PPy was synthesized by a similar method as the PPy–ZrP composites prepared without the ZrP particles. The condition of preparation and their ion-exchange capacity (IEC) of the cation exchange nanocomposite samples are given in Table 2.
Ion-exchange capacity (IEC)
The column method was used for the determination of the IEC of each sample; IEC generally expresses the measure of the H+-ion liberated by the nanocomposite cation exchanger to flow through the neutral salt. To calculate IEC 1 g of dry PPy–ZrP (in H+-form) was loaded into a glass column having an internal diameter ~1 cm with a glass wool supported at the bottom. The bed length was approximately 1.5 cm long. 1 Mol L−1sodium nitrate (NaNO3) as eluents was used to elute the H+ ions completely from the cation exchange column, keeping a very slow flow rate (~0.5 ml min−1). The effluent was titrated against a standard 0.1 M L−1 NaOH solution using phenolphthalein indicator. Table 2 shows the ion-exchange capacity values of the different samples.
Characterization
The Fourier transform infra-red spectroscopy (FTIR) spectra were recorded using Perkin Elmer 1725 instrument. Field emission scanning electron microscopy (FE-SEM) was used to study the surface morphology of the material using LEO 435-VF model electron microscope. Transmission electron microscopy (TEM) was performed by JEOL TEM (JEM 2100F) instrument. X-ray diffraction (XRD) data were recorded by PHILIPS PW1710 diffractometer with Cu Kα radiation at 1.540 Å in the range of 5° ≤ 2θ ≤ 70° at 40 kV. The thermal stability was investigated by thermal analysis (TGA, DTA and DTG) using thermal analyzer-V2.2A DuPont 9900. The samples were heated in alumina crucible from 30 to 1000 °C at the rate of 10 °C min−1 in the nitrogen atmosphere at the flow rate of 200 mL min−1. The elemental analysis of PPy, ZrP and PPy–ZrP cation exchange nanocomposite (PPy–ZrP) was performed using energy-dispersive analyzer unit (EDAX) attached with FE-SEM.
Electrical conductivity and ammonia-sensing measurements
For electrical conductivity measurements and sensing experiments, 0.2 g material from each sample was palletized at room temperature with the help of a hydraulic pressure instrument at 25 KN pressure for 10 min. DC electrical conductivity of the nanocomposite was measured using a four-in-line probe. The conductivity (σ) was calculated using the following equations [5–8]:
where G 7 (W/S) is a correction divisor which is a function of the thickness of the sample as well as probe-spacing where I, V, W and S are current (A), voltage (V), thickness of the film (cm) and probe spacing (cm), respectively. In isothermal ageing experiments, the nanocomposite pellets were heated at 50, 70, 90, 110 and 130 °C in a proportional integral directive (PID) controlled temperature oven. The electrical conductivity measurements were performed at an interval of 10 min. In cyclic ageing experiments, the DC electrical conductivity was measured in the temperature range of 40–150 °C repeatedly for five times at an interval of 1 h. Ammonia-sensing measurements were done by monitoring the resistivity of the nanocomposite using the Laboratory made set-up for ammonia sensing based on four-in-line probe electrical conductivity measuring instrument [6, 9].
Ammonia sensing kinetics
For sensing kinetics 0.2 g selected pelletized material was taken at 20 and 25 °C temperatures and resistivity response was recorded using four in line probe in atmosphere of ammonia vapours with respect to time.
Results and discussion
In this study various samples of PPy–ZrP cation exchange nanocomposite were prepared by in situ chemical oxidative polymerization of pyrrole in the presence of ZrP nanoparticles under different conditions (see Table 2). PZrP-7 sample shows better Na+ ion exchange capacity (1.60 meq g−1) as compared to the inorganic ZrP (sample ZrP-1, Table 1) (1.25 meq g−1). The IEC of PPyZrP composite was increased due to the addition of conductive polymer into the inorganic material (ZrP) which increases the surface area of the material; thus, exchangeable ionic sites were increased. Due to the better ion exchange capacity and electrical conductivity, sample PZrP-6 (Table 2) was selected for ammonia sensing.
The variation in conductivity with the loading of different amount pyrrole monomer is shown in Table 2. At 7% loading of pyrrole monomer high improvement in electrical conductivity and IEC was observed. Electrical conductivity increases significantly up to 5% loading of pyrrole monomer and decreases slightly at 7% loading. It means that by adding 5% PPy the percolation threshold might be achieved after further addition of PPy: no significant change in conductivity was observed. Since conductivity increases due to increase in concentration of conducting particles, it is to be well understood that conductivity depends significantly on the carrier transport through the conducting fillers. However, the formation of percolation network within the matrix of the composite also affects the conductivity. Thus after getting percolation threshold, further addition of PPy may change the network of the matrix and further addition of inorganic part (ion exchange material) to get better ion exchange capacity may also decrease the electrical conductivity due to its insulating property.
Temperature dependence of DC electrical conductivity of the PPy and PPy–ZrP
The electrical conductivity of PPy and PPy–ZrP cation exchange nanocomposite was measured with increasing temperatures from 30 to 150 °C. Arrhenius Plot (ln σ dc verses 1000/T) of electrical conductivity of PPy and PPy–ZrP cation exchange nanocomposite were obtained as shown in Fig. 1. Significant change in electrical conductivity of the nanocomposite was observed with the rise in temperature; electrical conductivity of PPyZrP increasing with increase in temperature can be explained by “thermal activated behaviour” [22]. The conduction mechanism in the conducting polymers is explained in terms of polaron and bipolaron formation. Polymer at low level of oxidation of the gives polaron and at high level of oxidation gives bipolaron. Both polarons and bipolarons are mobile and move along the polymer chain by the rearrangement of double and single bonds in the conjugated system. The mechanism of charge transport in polymer with non-degenerate ground state is mainly explained by conduction of polarons and bipolarons. The magnitude of the conductivity is dependent on the number of charge carriers available and their mobility. It has been observed that mobility of charge carriers increases with the increase in temperature leading to the increase in conductivity similar to that reported for PANI/WO3 and PANI/CeO2 nanocomposites [22, 23]. Another factor which also affects the electrical conductivity is the molecular alignment of the chains within the entire system.
FTIR studies
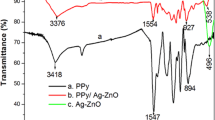
The FT-IR spectra of PPy, ZrP and PPy–ZrP nanocomposite are shown in Fig. 2. The FT-IR spectrum of PPy, in the fingerprint region of PPy, shows an absorption peak at 902 cm−1 which is characteristic of C–H out-of-plane deformation vibration, confirming the formation of PPy by the monomer. The bands at 1300, 3000 and 1500 cm−1 is attributed to the C–N in-plane, N–H starching and the bands at 1167 and 1041 cm−1 are related to the C–H bending modes while the strong absorption band obtained at 1449 and 1539 cm−1 corresponds to the C–C stretching and C=C bending vibration in the pyrrole ring. Some other peaks in the fingerprint region (600–1500 cm−1) can be attributed to the ring stretching and C–H in plane deformation mode. The PPy–ZrP nanocomposite shows nearly identical values and positions of the main IR bands in the range of 450–4000 cm−1. Compared with FTIR spectra of PPy, a strong band at 3400 cm−1 may be attributed to the –OH stretching frequency and a broad band between 1250 and 900 cm−1 with a peak of intensity at 1042 cm−1 is due to presence of ionic phosphate group and peak at 795 cm−1 is attributed to M–O bonding. The band at 3000 cm−1 is attributed to the N–H starching and the stretching vibration of C–N observed at 1325 cm−1 indicates that the polymerization of PPy has been successfully achieved on the surface of the ZrP milimicron particles.
X-ray diffraction studies
Figure 3 shows the XRD patterns of PPy, ZrP and PPy-ZrP nanocomposite. The XRD pattern of pure PPy shows an obvious broad peak at 2θ = 20°, along some very low intensity peaks, suggesting that the PPy conducting polymer is amorphous in nature [16]. XRD pattern of ZrP shows some sharp peaks at 2θ = 10°, 20°, 25°, 35° and some low intensity peaks [24]. In case of PPy–ZrP diffraction peaks lie between 15° and 25°, broaden slightly and low intensity of PPy at 34° and 36° appear in the XRD pattern of PPy–ZrP. These results suggest that PPy is polymerized and deposited on the surface of ZrP millimicron particles and there is the successful incorporation of ZrP nanoparticles in PPy–ZrP nanocomposite. It is also observed that the diffraction pattern of the nanocomposites slightly change as that of ZrP millimicron particles. Thus, we can conclude that PPy has low influence on the crystallization performance of ZrP millimicron particles. The results are also in agreement with the FTIR and TEM studies.
Morphological studies
Figure 4a and c shows the TEM image of ZrP and PPy–ZrP nanocomposite with tubular morphology having an average particle size of ~20–40 and 30–50 nm, respectively. Particle size of ZrP and PPy–TSP lies in nano range, which suggests that the prepared material is nanocomposite. The tubular ZrP nanoparticles can be seen as dark spots encapsulated in PPy matrix, which suggests that polymerization of PPy is successfully achieved on the surface ZrP nanoparticles. Figure 4b and d shows selected area diffraction pattern (SAED) of ZrP and PPy–ZrP nanocomposite predicts the crystalline nature of ZrP and semi-crystaline nature of PPyZrP nanocomposite.
The FE-SEM images of PPy, ZrP and PPy–ZrP nanocomposites are shown in Fig. 5a–c at different magnifications. Figure 5a shows globular nanoparticles of PPy, and Fig. 5b shows short tubular nanoparticles of ZrP. The FE-SEM images of PPy–ZrP nanocomposite (Fig. 5c) shows that ZrP nanoparticles are well embedded in the polymer matrix with uniform dispersion. Thus, the results of XRD, FTIR, TEM and SEM studies provided clear evidence that the polymerization of PPy has been successfully achieved on the surface of ZrP nanoparticles. A schematic representation of the formation of PPy and PPy–ZrP nanocomposite is given in Scheme 1.
Thermo gravimetric analysis
The TGA curve of PPy, ZrP and PPy–ZrP nanocomposite is shown in Fig. 6a. In case of PPy, first weight loss was observed at 200 °C (10.27%) due to physisorbed water molecule and volatile impurities. The second weight loss was observed at 400 °C (14.05%) due to degradation of the polymer unsaturated groups. After 250 °C there is gradual weight loss (31.11%) observed up to 1000 °C due to degradation of polymer [25]. In the case of ZrP, the first weight loss was observed at 100 °C (2.39%) due to removal of external water molecules, next on 500 °C (11.32%) due to starting of decomposition of the material and after 600 °C the ZrP was found stable up to 1000 °C. PPy–ZrP nanocomposite shows the first weight loss at 100 °C (6.87% weight loss) due to removal of external water molecules and after that PPyZrP is stable up to 500 °C. The second weight loss of PPy–ZrP appears at 550 °C (12.29% weight loss) because of degradation of PPy and after 600 °C the PPy–ZrP nanocomposite remains stable up to 1000 °C. The total mass loss up to 1000 °C has been estimated to be about 65.43, 11.62 and 28.68% for PPy, ZrP and PPy–ZrP, respectively. These results confirm that the presence of ZrP in PPy–ZrP nanocomposite is responsible for the higher thermal stability of the composite material in comparison to pristine PPy.
Figure 6b shows the DTA curve of pure PPy, ZrP and PPy–ZrP nanocomposite. DTA of ZrP was found to exhibit two endothermic peaks at 215 °C (2.22 μV) and 532 °C (0.30 μV) and one exothermic peak at 961 °C (−5.62 μV). The endothermic peaks at 215 °C corresponds to decomposition stage between 200 and 300 °C while the endothermic peak at 532 °C corresponds to second decomposition stage (400–600 °C). The exothermic peak corresponds to decomposition stage (900–1000 °C) also indicated in the TGA curve of ZrP (Fig. 6a). DTA of PPy was found to exhibit only one endothermic peak at 250 °C (2.22 μV), corresponds to decomposition stage between 200 and 300 °C as also indicated in TGA of PPy (Fig. 6a). However, PPy–ZrP exhibited two endothermic peaks at 121 °C (−4.96 μV) and 193 °C (−5.12 μV) corresponds to decomposition stage between (30–150 °C) and (150–300 °C), respectively, and one exothermic peak at 539 °C (1.43 μV) corresponds to decomposition stage between 400 and 650 °C as also indicated in TGA of PPy–ZrP nanocomposite (Fig. 6a).
DTG analysis of pure PPy, ZrP and PPy–ZrP nanocomposite was studied as a function of rate of weight loss (μg min−1) versus temperature (Fig. 6c). In case of pure PPy decomposition at 74 and 290 °C was found with 111 and 96 μg min−1 weight loss, respectively, and in case of ZrP decomposition at 109 and 529 °C was found with 94 and 529 μg min−1 weight loss, respectively. However, in the case of PPy–ZrP nanocomposite, the decomposition was observed at 64, 121,190, 539 °C with 100, 131, 166, 162 μg min−1 weight loss, respectively. Thus, it can be concluded from the DTG analysis that the rate of thermal decomposition is lower in case of PPy–ZrP, whereas in the case of PPy the rate of thermal decomposition was higher. The better thermal resistance of pure PPy–ZrP nanocomposite was due to incorporation of ZrP in the PPy matrix.
Energy-dispersive X-ray analysis
The EDAX patterns of PPy, ZrP and PPy–ZrP cation exchange nanocomposites are shown in Fig. 7. EDAX studies have provided clear evidence that the polymerization of PPy has been successfully achieved on the of the ZrP nanoparticles. The percent composition of elements is given in Table 3.
Stability in terms of DC electrical conductivity retention
The stability of the PPy and PPy–ZrP nanocomposite in terms of DC electrical conductivity retention was studied by isothermal ageing and cyclic ageing conditions in an ambient atmosphere.
DC electrical conductivity retention under isothermal ageing conditions
The isothermal stability of the composite material was examined in terms of DC electrical conductivity retention. In this experiment electrical conductivity was measured five times after an interval of 10 min at a particular temperature, e.g. 50, 70, 90, 110 and 130 °C in an air oven. Figure 8 shows electrical conductivity measurement with respect to time. It was observed that all the composite materials follow Arrhenius equation for the temperature dependence of the electrical conductivity from 50 to 90 °C and after that a deviation in electrical conductivity was observed, it may be due to the loss of dopant and degradation of materials. The isothermal stability of PPy–ZrP cation exchange nanocomposite in terms of DC electrical conductivity retention was found to be better than pristine PPy which suggests that the PPy–ZrP nanocomposite cation exchange may be used in electrical and electronic devices below 100 °C under ambient conditions.
DC electrical conductivity retention under cyclic ageing conditions
The stability of PPy and PPy–ZrP cation in terms of DC electrical conductivity retention exchange nanocomposite was also examined by cyclic ageing technique. It was observed from Fig. 9 that the DC electrical conductivity at the beginning of each cycle was found to be low as compared to previous cycle and which further decreases with the increase in number of cycles for PPy and PPy–ZrP cation exchange nanocomposite. This may be due to the loss of moisture and polymer degradation during cyclic ageing. From cyclic electrical conductivity study on PPy and PPy–ZrP cation exchange nanocomposite it may be suggested that the electrical conductivity of the PPy–ZrP nanocomposite is more stable than pristine PPy.
Ammonia vapour sensing characteristics of PPy–ZrP nanocomposite
The ammonia vapour sensing performance of PPy–ZrP (PZrP-6) cation exchange nanocomposite was monitored by measuring resistivity changes on exposure to ammonia vapours using laboratory-made assembly designed by using Four-in-line probe electrical conductivity device. The electrical resistance of nanocomposite showed remarkable changes on exposure to 0.2, 0.4, 0.6, 0.8 and 1 M concentrations of aqueous ammonia with vapour concentrations of 0.672, 0.870, 1.020, 1.080, and 1.097%, respectively, at room temperature as a function of time as depicted in Fig. 10a. It was observed that the nanocomposite showed a relatively fast response towards ammonia vapours in the concentration range of 0.2–1 M (vapour concentration 0.672–1.097%) and better resistivity response as compared to PPy (Fig. 10b); however, the change in resistivity on exposure to humidity was also observed as shown in the Fig. 10b. The relative humidity (%RH) inside the glass chamber was calculated as 82.93% by using the following relation [26]:
where E w (T1) (17.5 mm of Hg) and E w (T2) (21.1 mm of Hg) are the saturated water vapor pressure at the temperature of water (20 °C) and that of the composite film (23 °C), respectively. The values of the saturated vapor pressure were obtained from Lange’s handbook of chemistry by John A. Dean, Fifteenth Edition, Mc Graw-Hill, Inc. [27].Taking into account of some other ammonia-sensing composite materials like Polypyrrole graphitic nanocomposite [28], Polypyrrole/Metal Sulphide nanocomposite [29], poly(3-methythiophene)–titanium(IV) molybdophosphate cation exchange nanocomposite [6] and polyaniline–titanium(IV) phosphate cation exchange nanocomposite [8] reported earlier and compared with PPy–ZrP for ammonia sensing, the following observation can be noted (Fig. 10).
In Polypyrrole graphitic nanocomposite conductivity change in 25 min is 7%; and if it is calculated for PPy–ZrP it is 10.91% in 2 min. In the case of Polypyrrole/Metal Sulphide nanocomposite the response time for ammonia sensing is 20 s; on the other hand response time for PPy–ZrP is 10 Seconds. In the case of poly(3-methythiophene)–titanium(IV) molybdophosphate cation exchange nanocomposite, the change in resistivity is 0.06 Ω cm in 1.83 min, but in case of PPy–ZrP it is 0.35 Ω cm in 2 min. For polyaniline–titanium(IV) phosphate cation exchange nanocomposite change in resistivity is 0.8 Ω cm in 5 min and in the case of PPy–ZrP it is 0.35 Ω cm in 2 min. All the above results show that PPy–ZrP is a better sensor of Ammonia.
The resistivity was recovered on flushing with the ambient air. The response and recovery time of the sensor was around 10 and 30 s, respectively, for 0.2–1 M aqueous ammonia (vapour concentration 0.672–1.097%). The reversibility of the nanocomposite was also studied and the response of the nanocomposite was found to be highly reversible towards 0.2–0.6 M aqueous ammonia during the test of cyclic measurements as shown in Fig. 11. Further at higher concentration of aqueous ammonia (1 M), the reversible response studies were carried out which showed poor performance. The time taken to regain the resistivity value near to the original one was quite large. This poor performance of nanocomposite at higher concentration may be due to the complete occupying of reacting sites of polymer or because of the insufficient numbers of sites available for ammonia moiety to form the complex structure necessary for obtaining the response behaviour.
The extent of reversibility of the sensor was examined by cyclic measurements using different concentrations of ammonia (0.2, 0.6 and 1 M). The relative standard deviation (RSD %) for 0.2, 0.6 and 1 M was calculated to be 16.60, 1.56 and 1.01%, respectively. From the RSD (%) it can be concluded that the sensor works best in the concentration range from 0.2 to 0.6 M, and at higher concentrations slight irreversibility takes place which may be due to the electrical compensation of the polymer backbone by ammonia.
Second-order kinetics evaluation for ammonia sensing on PPy–ZrP nanocomposite and kinetic parameters
Order of reaction of ammonia vapour sensing on PPy–ZrP nanocomposite was evaluated for the physical interaction of ammonia on PPy–ZrP nanocomposite. The mechanism of interaction may be explained on the basis of the electrostatic interaction of the lone pair of nitrogen of ammonia with carbon in PPy of PPy–ZrP nanocomposite. To ascertain the order of the reaction, the standard equation for first and second were applied as given below:
where σ is the conductivity (reverse of resistivity) response recorded during sensing, σ 0 is the conductivity at the start of the sensing, k 1 is the rate constant and t is the time.
A tentative explanation of processes occurring on the surface of PPy–ZrP can be explained as the lone pair of nitrogen of ammonia interacts with the carbon of PPy, which decreases the intensity of positive charge and hence the mobility of charge carriers decreases resulting in the decrease in conductivity. The mechanistic representation of the electrical compensation of PPy in the PPy–ZrP nanocomposite in the present work is given in Scheme 2.
Conductivity vs. time and reverse of conductivity vs. time graph for sensing of ammonia vapours on PPy–ZrP in Fig. 12a and b shows straight line pattern in which Fig. 12b most resembles the second-order reaction.
Rate constants K 1 (0.0027 L Mol−1 S−1) and K 2 (0.0029 L Mol−1 S−1) at 20 and 25 °C were determined from the slopes of the graph of the inverse of conductivity verse time in Fig. 13.
According to the transition state theory presented by Laidler [30] the rate constant for a process can be written as
where K B is Boltzmann’s constant, h is Plank’s constant, T is absolute temperature, ΔS* is the entropy of activation, ΔH* is the enthalpy of activation and R is the gas constant.
The Arrhenius activation energy, E a is determined from the Arrhenius equation at two different temperatures.
The enthalpy of activation, ΔH*, can be calculated from the relationship:
The entropy of activation ΔS* was calculated from the Eq. (8) and the free energy of activation ΔG* was determined from
Results are summarized in Table 4 the negative value of ΔS* and positive value of ΔH* indicate the feasibility and endothermic behaviour during sensing process.
Sensing mechanism
The vapour-sensing properties of PPy–ZrP with ammonia vapours can be explained by the interaction of ammonia with PPy in the PPy–ZrP nanocomposite and is almost a reversible process, although a little bit of irreversibility is observed. Under ambient conditions, the value of resistivity increased due to the interaction of lone pair electron of ammonia with the positive charge of PPy in PPy–TSP. The mobility of charge carriers decreases which leads to an increase in resistivity. The interaction between lone pair of ammonia and positive charge of polarons or bipolarons of PPy in PPy–ZRP is electrostatic because it occurs between two opposite charges. Due to the small magnitude of charge, this interaction is weak in nature so it becomes reversible when it comes in contact with air, thus resistivity is restored. The resistivity did not come back to its original value and was always found higher than the previous value for higher concentration of ammonia. Hence, it can be concluded that there are two processes in operation: first, reversible chemisorption of ammonia with PPy occurs and second, compensation or electrical neutralization of the polymer backbone takes place.
In the light of observation by Khan [31] in polypyrrole–titanium(IV) sulphosalicylophosphate nanocomposite cation exchange material, it can be inferred that the lone pair of acetaldehyde interacts with positive charge of PPy, which decreases the intensity of positive charge and hence the mobility of charge carriers decreases resulting in the increase in resistivity. Since the exposure to ammonia was carried out in a closed system and chemical linking is much more complicated process, desorption of ammonia also occurs readily under ambient conditions and thus the resistivity is restored.
On exposure to ammonia for long duration, complete electrical neutralization of the polymer backbone occurred. The mechanistic representation of the electrical compensation of PPy in the PPy–ZrP nanocomposite in the present case is shown in Scheme 2a and b.
Conclusion
In the present study, the PPy–ZrP cation exchange nanocomposites have been synthesized by in situ chemical oxidative polymerization technique. The results of TEM, SEM, XRD and FTIR studies reveal that the polymerization of pyrrole has been successfully achieved on the surface of the ZrP particles and indicates that there is a strong interaction between PPy and ZrP nano particles. The PPy–ZrP cation exchange nanocomposites show improved thermal stability, isothermal stability in terms of DC electrical conductivity as well as ion exchange capacity in comparison with the pure PPy. The reproducible ammonia sensing results in the range of 0.2–0.6 M aqueous ammonia indicates that PPy–ZrP nanocomposite can be used in making a sensing device.
Sensing kinetics of ammonia vapour on PPy–ZrP was also studied and it was found that ammonia vapour sensing on PPy–ZrP follow the second-order of kinetics.
References
Kim BH, Park DH, Joo J, Yu SG, Lee SH (2005) Synthesis, characteristics, and field emission of doped and de-doped polypyrrole, polyaniline, poly (3, 4-ethylenedioxythiophene) nanotubes and nanowires. Synth Met 150:279–284
Mahmoudian MR, Alias Y, Basirun WJ (2010) Electrodeposition of (pyrrole-co-phenol) on steel surfaces in mixed electrolytes of oxalic acid and DBSA. Mater Chem Phys 124:1022–1028
Asan A, Kabasakaloglu M, Aksu ML (2005) The role of oxalate ions in the coverage of mild steel with polypyrrole. Russ J Electrochem 41:175–180
Wei L, Hu N, Zhang Y (2010) Synthesis of polymer—mesoporous silica nanocomposites. Materials 3:4066–4079
Khan AA, Baig U (2014) Electrical and thermal studies on poly(3-methyl thiophene) and in situ polymerized poly(3-methyl thiophene) cerium(IV) phosphate cation exchange nanocomposite. Compos Part B 56:862–868
Khan AA, Baig U (2013) Electrical conductivity and ammonia sensing studies on in situ polymerized poly(3-methythiophene)–titanium(IV) molybdophosphate cation exchange nanocomposite. Sens Actuators, B 177:1089–1097
Khan AA, Baig U (2011) Ammonia vapor sensing properties of polyaniline–titanium(IV) phosphate cation exchange nanocomposite. J Hazard Mater 186:2037–2042
Shakir M, Iram NE, Khan MS, Al-Resayes SI, Khan AA, Baig U (2014) Electrical conductivity, isothermal stability, and ammonia-sensing performance of newly synthesized and characterized organic-inorganic polycarbazole–titanium dioxide nanocomposite. Ind Eng Chem Res 53:8035–8044
Yuvaraj H, Woo MH, Park EJ, Jeong YT, Lim KT (2008) Polypyrrole/gamma Fe2O3 magnetic nanocomposite synthesized in superficial fluide. Eur Polym J 44:637–644
Nandi D, Gupta K, Ghosh AK, De A, Ray NR, Ghosh UC (2013) Thermally stable polypyrrole–Mn doped Fe(III) oxide nanocomposite sandwiched in graphene layer: synthesis, characterization with tunable electrical conductivity. Chem Eng J 220:107–116
Patil DS, Pawar SA, Devan RS, Gang MG, Ma YR, Kim JH, Patil PS (2013) Electrochemical supercapacitor electrode material based on polyacrylic acid/polypyrrole/silver composite Electrochim Acta 105:569–577
Wang S, Jiang SP, White T, Guo J, Wang X (2009) Electrocatalytic activity and interconnectivity of Pt nanoparticles on multiwalled carbon nanotubes for fuel cells. J Phys Chem C 113:18935–18945
Wang B, Li C, Pang J, Zhai J, Li Q (2012) Novel polypyrrole-sensitized hollow TiO2/fly ash cenospheres: synthesis, characterization, and photocatalytic ability under visible light. Appl Surf Sci 24:9989–9996
Peng S, Wu Y, Zhu P, Thavasi V, Mhaisalkar SG, Ramakrishna S (2011) Facile fabrication of polypyrrole/functionalized multiwalled carbon nanotubes composite as counter electrodes in low-cost dye-sensitized solar cells. J Photochem Photobiol A Chem 223:97–102
Cervantes SA, Roca MI, Martinez JG, Olmo LM, Cenis JL, Moraleda JM, Otero TF (2012) Bioelectrochemistry 85:36–43
Hong L, Li Y, Yang M (2010) Fabrication and ammonia gas sensing of palladium/polypyrrole nanocomposite. Sens Actuators B Chem 145:25–31
Nowicka AM, Fau M, Rapecki T, Donten M (2014) Polypyrrole-Au nanoparticles composite as suitable platform for DNA biosensor with electrochemical impedance spectroscopy detection. Electrochim Acta 140:65–71
Deivanayaki S, Ponnuswamy V, Mariappan R, Jayamurugan P (2013) Synthesis and characterization of polypyrrole/TiO2 composites by chemical oxidative method. Optik 124:1089–1091
Grover S, Shekhar S, Sharma RK, Singh G (2014) Multiwalled carbon nanotube supported polypyrrole manganese oxide composite supercapacitor electrode: role of manganese oxide dispersion in performance evolution. Electrochim Acta 116:137–145
Khan AA, Baig U (2012) Polyacrylonitrile-based organic-inorganic composite anion-exchange membranes: preparation, characterization and its application in making ion-selective membrane electrode for determination of As(V). Desalination 289:21–26
Khan AA, Baig U (2012) Electrically conductive membrane of polyaniline–titanium(IV) phosphate cation exchange nanocomposite: applicable for detection of Pb(II) using its ion-selective electrode. J Ind Eng Chem 18:1937–1944
Parvatikar N, Jain S, Bhoraskar SV, Prasad MVNA (2006) Spectroscopic and electrical properties of polyaniline/CeO2 composites and their application as humidity sensor. J Appl Polym Sci 102:5533–5537
Parvatikar N, Jain S, Khasim S, Revansiddappa M, Bhoraskar SV, Prasad MVNA (2006) Electrical and humidity sensing properties of polyaniline/WO3composites. Sens Actuators B Chem 114:599–603
Sun L, Boo WJ, Sue HJ, Clearfield A (2007) Preparation of α-zirconium phosphate nanoplatelets with wide variations in aspect ratios. New J Chem 31:39–43
Bose S, Kuila T, Uddin ME, Kim NH, Lau AKT, Lee JH (2010) In-situ synthesis and characterization of electrically conductive polypyrrole/graphene nanocomposites. Polymer 51:5921–5928
Khan AA, Khalid M, Niwas R (2010) Humidity and ammonia vapor sensing applications of polyaniline–polyacrylonitrile composite films. Sci Adv Mater 2:474–480
Dean JA (1998) Lange’s handbook of chemistry, 15th edn. Mc Graw-Hill Inc, pp 528–529
Jang WK, Yun J, Kim HI, Lee YS (2013) Improvement of ammonia sensing properties of polypyrrole by nanocomposite with graphitic materials. Colloid Polym Sci 291:1095–1103
Yeole B, Sen T, Hansora D, Mishra S (2016) Polypyrrole/metal sulphide hybrid nanocomposites: synthesis, characterization and room temperature gas sensing properties. Mater Res 19:999–1007
Laidler KJ (1965) Chemical kinetics. McGraw-Hill, New York, p 556
Khan AA, Hussain R, Shahen S (2016) Electrical conductivity and acetaldehyde vapour sensing studies on synthetic polypyrrole–titanium(IV) sulphosalicylophosphate nanocomposite cation exchange material. New J Chem 40:2200–2210
Acknowledgements
Authors are thankful to the University Grant Commission (India) for MANF and Department of Applied Chemistry for providing financial support and research facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Khan, A.A., Hussain, R. & Baig, U. DC electrical conductivity and rate of ammonia vapour-sensing performance of synthetic polypyrrole–zirconium(IV) phosphate cation exchange nanocomposite. Int J Ind Chem 8, 157–173 (2017). https://doi.org/10.1007/s40090-017-0118-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40090-017-0118-z