Abstract
Acetylation of glycerol with acetic acid was carried out over bimetallic silver and copper deposited rice husk silica-alumina like ecofriendly green catalyst. Bio-additive like mono, di and tri acetyl glycerol synthesis from raw glycerol (one of the main product of biodiesel), which are gaining attention as additives for improving petroleum fuel properties towards biofuel additive development applications. Advantage of using bimetallic catalyst for glycerol acetylation due to possible synergistic effect between the metals and it enhances the catalytic conversion and selectivity compared to single metal catalyst. The prepared catalysts were characterised by XRD, FT-IR and TEM. Silver and copper incorporated RHS (rice husk silica)-alumina catalysts are shown higher activity and selectivity towards diacetin (di acetyl glycerol) and triacetins (tri acetyl glycerol) formation by catalytic acetylation of glycerol. Higher conversion (98 %) and good selectivity (51 %) is achieved.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glycerol is a valuable by product during biodiesel production (10 % in weight). The utilization of glycerol for the synthesis of value added chemical is a topic of great industrial interest because glycerol can be formed in large amounts during the biodiesel production and represents as a waste byproduct. Its effective utilization will be a key factor that can promote biodiesel commercialization and further development. One of the most attractive outlets of glycerol is to produce glycols especially propanediols by selective hydrogenolysis of glycerol [1, 2]. This process provides a clean and economically competitive route for the production of these commodity chemicals from renewable glycerol instead of nonrenewable petroleum products. Consequently, many efforts have been attempted to facilitate this important reaction such as supported noble metals like Ru, Rh, and Pt are well-known active catalysts in the hydrogenolysis of glycerol [3]. Unfortunately, these catalysts are often promoting excessive C–C cleavage, resulting in a low selectivity to propanediols. As a less expensive alternative, copper-based catalysts have been reported to have a superior performance in this reaction due to their poor activity for C–C bond cleavage and high efficiency for C–O bond hydro-dehydrogenation [4, 5].
Karinen and Krause reported the etherification of glycerol with isobutene over acid exchange resin catalyst [6, 7]. Etherification of glycerol with tert-butanol over acid exchange resin or zeolite catalysts are also reported [8, 9]. Reddy et al. [10], and coworkers reported sulphated ceria-zirconia and ceria alumina type solid acid catalyst for glycerol acetylation to replace mineral acid based catalyst. Bagheri and Muhd [20], reviewed the various value added product formation from glycerol by bimetallic catalysts. Hence, utilization of glycerol by acetylating agent via catalytic method is another alternative methodology. The present study deals with bimetallic particle doped silica-alumina type solid acid catalyst preparation and tested for glycerol acetylation. The glycerol acetylaiton products such as mono, di and triacetyl esters have great industrial applications. The triacetylated derivative is known as triacetin and has application going from cosmetics to fuel additive [11, 12]. The mono and diacetylated esters (monoacetin and diacetin) are used in cryogenics and as raw material for production of biodegradable polyesters [13]. The aim of the present work deal with acetylation of glycerol over silver and copper incorporated rice husk silica-alumina solid acid catalyst to produce di and triacetylated glycerols with good selectivity under mild reaction conditions. The influence of various reaction parameters has also been studied.
Experimental
Catalyst preparation
Rice husk was collected from a rice mill in Penang, Malaysia. The rice husk silica was prepared by our previously reported procedure [14]. After washing and rinsing the rice husk (RH) several times with distilled water it was dried at room temperature. About 30.0 g of clean RH was stirred in 750 mL of 1 M HNO3 at room temperature for 24 h to remove all metallic impurities and this acid treated RH was washed with distilled water until the pH of the rinse was constant (around 4.8–5.0) and dried in an oven at 373 K for 24 h, further kept in a muffle furnace at 873 K for 6 h for complete combustion. The white rice husk silica ash (RHA) thus obtained was used as source of silica for further studies.
About 10 g of the RHA was stirred continuously in 100 mL of 3 M NaOH to obtain the sodium silicate solution. To the obtained sodium silicate solution, Al (NO3)3·9H2O (Si:Al = 1:1) and cetyl trimethyl ammonium bromide (CTAB) were added and precipitated at pH = 12.3. The solid obtained was washed, dried at 383 K and calcined at 873 K. The xerogel obtained was ground to powder and labelled as RHS-Al. Similar procedure was repeated with the addition of Cu(NO3)3·3H2O to prepare Cu (10 wt%) doped RHS-Al catalyst (10Cu/RHS-Al). To this system appropriate amount of AgNO3 was added and precipitated to obtain different amount of silver-copper deposited RHS-Al materials (1 %Ag–10 %Cu/RHS-Al and 5 % Ag–10 %Cu//RHS-Al).
Characterization
The powder X-ray diffraction pattern of the catalysts was collected on Siemens Diffractometer D5000, Kristalloflex operated at 40 kV and 10 mA with nickel filtered CuKα radiation, c = 1.54 Å. The N2-sorption data and BET values were collected on NOVA 2200 type surface area and pore size analyzer. Pyridine adsorption analysis carried out by gaseous adsorption method using conventional hammet method. The FT-IR analysis was carried out on Perkin-Elmer System 2000 using KBr pellet method. The morphology and elemental analysis characterized by energy dispersive x-ray spectrometry (Edax Falcon System) and transmission electron microscopy (Philips CM12).
Acetylation reaction
The acetylation of glycerol was carried out in a 50 mL double neck round bottom flask connected with a reflux condenser kept in an oil bath, temperature of which was controlled by a thermocouple. In a typical reaction, the catalyst powder (80 mg, dried at 383 K), was suspended in a mixture of glycerol and acetic acid (1:10) and kept in an oil-bath at the required reaction temperature (383 K). The system was subjected for stirring (700 rpm). About 5 µm of solution was withdrawn at regular intervals period (30 min) and analysed by GC using toluene as an internal standard. The products were analysed on Clarus 500 (PerkinElmer) gas chromatograph with a capillary column, Elite Wax (30 m length and 0.32 mm inner diameter) equipped with an FID detector using toluene as the internal standard. The set temperature programme was 323 K/3 min-temperature ramp of 283 K/min to 503 K with N2 as carrier gas. Products were confirmed on GC–MS in Elite Wax column equipped with a mass selective detector.
Results and discussion
Characterisation of Ag–Cu–silica–alumina catalysts
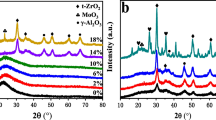
The wide-angle powder X-ray diffraction pattern of as prepared catalysts with parent rice husk silica is shown in Fig. 1. The pattern depicts that RHS-Al is amorphous in nature with a broad peak in the 2θ region of 20–30° at the calcination temperature of 773 K [16]. During copper incorporation in rice husk silica results in the formation of CuO nanoparticle and crystallized in monoclinic structure, which is confirmed by XRD pattern. The reflection observed at 35.5° and 38.7° corresponds to the formation of CuO nanoparticle phase formation [PDF 05-0661]. Similar observations are obtained for CuO-niobia/silica-alumina catalyst [17]. Small amount of silver particle incorporated on Cu/RHS-Al is not impact the crystal phase changes in the XRD pattern suggesting that the silver particle dispersion on the support and EDX analysis confirm the existence of Ag and Cu by elemental analysis. Nitrogen adsorption and desorption study is rice husk silica and silver, copper doped rice husk silica are shown in Fig. 2. The bimetallic particle incorporated rice husk silica shown the similar characteristic curve related with micro porous structure. Figure 3 shows the FT-IR spectra of representative samples recorded at 400–4000 cm−1 rang and in all spectra, a broad absorption band appeared around ~3456 and 1635 cm−1 corresponding to the vibrations of –OH and silanol group [14, 15]. The strong bands at ~1011 cm−1 can be assigned to typical Si–O–Si vibration. Bands at ~708 and 452 cm−1 are due to the Si–O vibration within Si–O–Si [14]. FT-IR spectra of samples after pyridine-adsorption were recorded to study the nature of acidity exist in the catalyst (Fig. 3). For all catalysts, no prominent peaks were observed as seen in the spectra. In the entire sample a broad absorption band was observed at ~1650 cm−1 corresponding to bronsted acid nature of the support [15]. In the case of RHS-A1 exhibits a band centred at ~1575 cm−1 due to surface hydroxyl acidity. Therefore, bronsted acidity in nature exists predominantly in the as prepared Ag–Cu/RHS-Al solid acid catalyst. Morphologies of as synthesized bimetallic Cu–Ag-rice husk silica-alumina catalyst are shown in Fig. 4. The magnification of Fig. 4a is around 5 µm. The SEM images shown the aggregated particle morphology for as prepared bimetallic solid acid catalyst and copper oxide nanoparticle forms the cubic shape particle on RHS-alumina support, which is shown in arrow mark in Fig. 4a. The white colour cubic particle exists on silica-alumina due to formation of crystallized copper oxide particle after calcinations process (Fig. 4b). The amount of copper and silver is further determined by elemental analysis using EDX connected with SEM equipment. Figure 5 shows the elemental composition of as prepared bimetallic rice husk silica catalyst; edx spectra confirm the copper and silver particle existence in RHS support. TEM images are also confirms the aggregated particle morphology formation for as prepared solid catalysts and the size of the particle exists in the range of 30–40 nm and the scale value in the TEM image is 50 nm. The dense lengthy particle in the TEM image of Fig. 3b is due to existence copper oxide nanoparticle aggregation with RHS-alumina support. The length of the dense particle is more than 50 nm and below 80 nm. The pore size N2 sorption analysis shows the uniform porosity and BET surface area of 1Ag–5Cu/RHS-Al to RHS-Al are shown in Fig. 2 and Table 1.
Catalytic activity of Cu–Ag–silica–alumina catalysts
Acetylation of glycerol over different amount of copper and silver incorporated rice husk silica catalyst is shown in Table 2. Silver and copper incorporated in the ratio of 1:10 and the reaction carried out at various temperatures. Conversion of glycerol acetylation over 1Ag–10Cu/RHS-Al catalyst is increased upon increase with reaction temperature. At higher temperature like 383 and 393 K shows the maximum conversion towards glycerol derivative formation. The selectivity towards monoacetin was decreased and diacetin and triacetin formation was increased. Tiracetin formation is increased at optimized temperature range between 353–373 K and above 373 K, triacetin formation was slashed. The selectivity of diacetin formation is very low at starting temperature and increased upon optimized reaction temperature. The di and tri-acetin formation was improved in the presence of bimetallic Ag–Cu loaded silica-alumina catalyst at optimized reaction condition.
The effect of molar ratio between glycerol and acetic acid has also been studied in the presence of 1Ag10Cu-silica-alumina catalyst. The results of glycerol acetylation with different mole ratio of acetic acid and glycerol were shown in Table 3. In all analysis toluene is used as internal standard for determination of glycerol conversion in GC analysis. A Table 4 show the higher amount of silver incorporation on Cu/RHS-A1 solid catalyst is increases the glycerol conversion and selectivity towards di and triacetin formation. Silver incorporation is acting as a promoter for copper-RHS-A1 catalyst and increasing the metal particle deposition results in the higher amount of glycerol conversion occurred. From Table 3, Glycerol conversion increased gradually with increased amount of acetic acid, results in the formation of higher yield of diacetin and triacetin. At higher mole ratio between glycerol/acetic acid is hinder the formation of monoacetin and higher selectivity obtained for triacetin formation in the presence of Ag–Cu–silica–alumina bimetallic catalyst. Table 3 shows maximum conversion (80.4 and 85.7 %) with the higher selectivity towards triacetin formation (30.1 and 43.2 %) at increase amount of mole ratio such as 1:20 and 1:25. In eco-point of view and cost effective in large scale synthesis is optimized towards 1:10 more ratio usage of glycerol: acetic acid is the best for obtain MAG. Table 4 show the higher glycerol conversion with good selectivity at optimized glycerol and acetic acid mole ratio (1:10). Hence, bimetallic nanoparticle deposition on silica support and mole ratio variation between glycerol and acetic acid are playing important role in triacetin formation from glycerol. Addition of appropriate amount of silver and copper deposition on silica-alumina catalyst shows the considerable improvement in catalyst texture property towards high catalytic activity. The higher amount of bimetallic species incorporated catalysts (1Ag10Cu/RHS-Al and 5Ag10Cu/RHS-Al) shown the 100 % conversion for catalytic glycerol conversion and good product selectivity obtained towards diacetin and triacetin formation (Table 4). Figure 6 shows the pictorial representation of glycerol conversation and clear image of selectivity towards triacetin formation at higher mole ratio of substrate to reactant. One can envisage two possible mechanisms [18] for acetylation in strong acidic medium: the first one is the normal AAC2 mechanism, involving protonation of the carbonyl oxygen atom and nucleophilic attack in the carbonyl to form a tetrahedral intermediate, presenting a quaternary carbon atom (Scheme 1); the second mechanism is the AAC1, where protonation takes place in the oxygen atom attached to the carbonyl group, followed by formation of an acylium ion (Scheme 2). The first pathway is normally less energetic, because of the higher stability of the intermediate formed upon protonation in the carbonyl oxygen atom. On the other hand, formation of the tetrahedral intermediate is space demanding, and the second mechanism, involving the acylium ion, prevails in situations of steric constraints [19].
Table 5 shows the comparative catalytic activity of solid catalysts prepared by low cost methodology and conventional route prepared catalyst. The standard solid acid catalyst such as K-10, Niobium phosphate and Amberlyst 15 are shown complete conversion for glycerol acetylation with less selectivity towards triacetin formation. In our case, the prepared bimetallic catalysts like Ag–Cu–silica–alumina are showing higher selectivity towards diacetin (DAG) and triacetin (TAG) formation. The DAG and TAG are good additives for fuels such as biodiesel and gasoline due to their role in improve the viscosity. Another advantage of the present work is low cost methodology was adopted to prepare the bimetallic catalysts. The application of triacetin is very much vital for cosmetics formation and also used as additives for petroleum products.
Conclusions
Rice husk silica-alumina and bimetallic Ag–Cu/RHS-Al solid acid catalysts were prepared by low cost methodology via sol–gel method and characterized by various physico-chemical techniques. Mono, Di, Triacetins are obtained as major products in the presence of Ag–Cu/RHS-Al catalyst and negligible conversion rate was obtained on raw RHS catalyst. The rod like aggregated particle morphology obtained for Ag–Cu/RHS-Al catalyst. Ag–Cu modified rice husk silica-alumina catalyst shows higher selectivity towards DAG and TAG formation compared to other conventional catalysts. Conversion and selectivity for glycerol acetylation reaction are improved greatly due to nature of active centers exists on bimetallic-rice husk silica catalyst.
References
Dasari MA, Kiatsimkul P-P, Sutterlin WR, Suppes GJ (2005) Appl Catal A Gen. 281:225
Maris EP, Ketchie WC, Murayama M, Davis RJ (2007) J Catal 251:281
Feng J, Fu HY, Wang JB, Li RX, Chen H, Li XJ (2008) Catal Commun 9:1458
Runeberg J, Baiker A, Kijenski J (1985) Appl Catal. 17:309
Montassier C, Giraud D, Barbier J (1988) In: Guisnet M, Barrault J, Bouchoule C, Dupres D, Montassier C, Pe´rot G (eds.) Stud. Surf. Sci. Catal. Amsterdam: Elsevier, pp. 165
Mat R, Amin NAS, Ramli Z, Abu Bakar WA (2006) J Nat Gas Chem 15: 259
Karinen RS, Krause AOI (2003) Appl Catal A Gen. 306:128
Wessendorf R, Erdoel Kohle (1995) Erdgas Petrochemie 48: 138
Klepacova K, Mravec D, Bajus M (2005) Appl Catal A 294:141
Reddy PS, Sudarsanamm P, Raju G, Reddy BM (2012) J Indus Eng Chem. 18:648
Nomura S, Hyoshi T (1995) JP patent 203429
Lipkowski AW, Kijenski J, Walisiewicz-Niedbalska N (2005) Pol Chemik 58:238
Taguchi Y, Oishi A, Ikeda Y, Fujita K, Masuda T (2000) JP patent 298099
Adam F, Radhika T (2010) Chem Eng J 160:249
Muruvvet Y, Mehmet A, Tonbul Y, Kadir Y (1999) Turk J Chem 23:319
Fillipe AC Garcia, Valdeilson S. Braga, Júnia CM Silva, José A. Dias, Sílvia CL Dias, Jorge LB Davo (2007) Catal Lett 119:101
Silva LN, Gonçalves VLC, Mota CJA (2010) Catal Commun 11:1036
Lowry TH, Richardson KS (1981) Mechanism and theory in organic chemistry, 2nd edn. Harper and Row, New York, pp 652–658
Bender ML, Ladenheim H, Chen MC (1961) J Am Chem Soc 83:123–127
Bagheri S, Muhd Julkapl N, Yehye WA (2015) Renew Sustain Energy Rev 41:113–127
Acknowledgments
The research project was financially supported by King Saud University, Deanship of Scientific Research, Research Chairs.
Authors Contributions
RJ performed all experiments, processed data, initial data analysis and first draft of manuscript; HAA guidance, TR secondary data analysis, subsequent drafts of manuscript FA, guidance, THD project design. All authors have read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Jothi Ramalingam, R., Radhika, T., Adam, F. et al. Acetylation of glycerol over bimetallic Ag–Cu doped rice husk silica based biomass catalyst for bio-fuel additives application. Int J Ind Chem 7, 187–194 (2016). https://doi.org/10.1007/s40090-016-0073-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40090-016-0073-0