Abstract
Mixed matrix membranes prepared by varying compositions of Cellulose acetate, acetone and formamide for the synthesis of ultrafiltration/nanofiltration membranes with and without nanoparticles for the removal of arsenic from synthetic solution. Zinc oxide nanoparticles were synthesized by an in situ ultrasonic technique and characterization done using XRD and SEM. In the current study, batch experiments were conducted to characterize the maximum removal efficiency of arsenic by cellulose acetate–ZnO mixed matrix membrane. It was found that 58.77% of arsenic removal was obtained for the feed concentration of 1000 mg/L and pH range 6.8 ± 0.6. The nanoparticle-embedded membranes show higher removal efficiency, high flux and permeation rate than cellulose acetate membranes without embedded nanoparticles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Arsenic is a naturally occurring contaminant, immense concern for the environment and human health because they are highly toxic and present in many contaminated sites, causing an adverse impact on human as well as animal health. Water treatment using membranes is the most popular technology adopted today. Globally, the high levels of arsenic in drinking water are a serious problem [26]. Most of the rural population in India rely on the groundwater sources for drinking purposes, which generally contain underground deposits such as salts and minerals particularly in the West Bengal, India and Bangladesh [8, 10, 34, 38, 39, 47, 63] have stated that the contamination of groundwater by arsenic [54]. The arsenic (As) occurs in different oxidation states as As(0), As(III), As(V), and As(-III) [9, 13, 14, 24, 57]. However, in oxidizing conditions arsenic appears as oxyanions [12, 46]. In most of the cases, As(III) is 30 times on an average more toxic than As(V) [9, 31]. In most of the countries, Arsenic in groundwater less than 10 μg/L is safe. High concentrations of As are found due to reducing and oxidizing aquifers besides areas affected by industrial, mining and geothermal action [16, 62]. Long-term drinking water exposure leads to lung, skin, kidney and bladder cancer in addition to pigmentation changes, neurological disorders, nausea, hyperkeratosis, muscular weakness and loss of appetite [4, 24, 35].
Conventional treatment methods for the removal of contaminants from groundwater include adsorption, precipitation, electrochemical oxidation and chemical, biological degradation, foam fractionation and membrane technology [1, 50]. Membrane plays a vital role in terms of separation of desired components [3, 48]. As a result, heavy metal separation by membrane filtration attained importance [18, 22, 23]. Development of membranes is a key area of research to synthesize most durable and the high-performance membranes [29, 30]. However, due to the limitations of existing membranes, much research is going on for the synthesis of novel membranes [69, 70] as a thin barrier between miscible fluids [19]. During the separation, a suitable pressure or concentration differential is applied across the membrane [20]. Pressure-driven [2] membrane processes can be classified by several criteria like membrane pore size, the pressure exerted on the membrane and charge of the membrane as well as depend on physical properties of the membrane, such as thickness, hydraulic permeability in addition system variables such as filtration time, initial concentration and transmembrane pressure [6, 49, 55, 58]. Nanoparticles have attracted to the amendment of asymmetric mixed matrix membranes with various methods. Using phase inversion processes to fabricate the asymmetric membranes [37], various researchers directly mixed/blended the prepared nanoparticles into the membrane casting solution [5, 7, 15, 53, 65, 67]. The addition of inorganic nanoparticle enhances the hydrophilic and permeability rates of the membranes and shows antimicrobial activity [41, 61, 66]. The incorporated nanoparticles also affect the charge of the membrane, thermal stability, mechanical strength and the membranes showing the antiviral and anti-bacterial properties and also improve the discerning separation, specifically protein separation [27, 45, 59].
In the current study, the inorganic nanoparticles were synthesized using ultrasonication techniques which play in the formation of nanosize particles as reported [59]. Zinc oxide nanoparticles play an important role in antimicrobial, anti-fungal properties and biofouling [17, 21, 51, 56] and nanoparticles are characterized by XRD and SEM [36]. CA/ZnO asymmetric mixed matrix membranes are synthesized using the phase inversion technique [43, 53, 59] and performance analyses of the CA/ZnO membranes like pure water flux, hydraulic permeability, and removal efficiency were analyzed [59].
Materials and methods
Materials
Cellulose acetate (CA) extra pure with acetyl content ranging from 29 to 45% from lobachemie, Formamide pure, and acetone (purity 99%) was obtained from SRL chemicals which were used as received to prepare the casting solution. Zinc chloride, sodium hydroxide Pure, and PEG 6000 were used to prepare the zinc oxide nanoparticles. Arsenic trioxide extra pure is used to prepare the synthetic solution. Arsenic stock solution was prepared by dissolving 1.320 g in 1 L of deionized water giving a concentration of 1000 mg/L with mild heating.
Synthesis of Zinc oxide nanoparticles
Initially, polyethylene glycol (PEG: molecular weight ≈ 6000) and deionized water have been dissolved in 4:1 ratio and allowed on a magnetic stirrer. ZnCl2 and NaOH [28, 42] with molar ratio 1:1 were dissolved in one deionized water in separate beakers. In the presence of ultrasound, the prepared PEG and ZnCl2 solutions are allowed to mix for 1 h. NaOH solution was added to the PEG + ZnCl2 solution mixture drop by drop in the presence of ultrasonication. As a result of heating, crystal nuclei were formed, which then grow as a white precipitate of Zn(OH)2 was formed. It was filtered and rinsed several times with deionized water until the PEG and NaCl were washed away and the precipitate was dried in a hot air oven at 80 °C for 48 h.
Preparation of cellulose acetate/nanocomposite membranes
The CA asymmetric mixed matrix flat sheet membranes and unmodified CA membranes were prepared in the laboratory by phase inversion solvent evaporation method as described [33, 52] and the casting solution details are listed in Table 1. In the presence of ultrasonication, the zinc oxide nanoparticles were added/dispersed in the casting solution as listed in Table 1. Followed by the casting solutions were kept idle without stirring for 5 min to remove air bubbles. Using a casting/bar coater, the viscous liquid formed has been used to cast the membrane and the casting conditions are given in Table 2.
Characterization of nanoparticles and CA membranes
Nanoparticles have been analyzed using the XRD analyzer (PAN analytical’s Xpert Powder) with the parameters: step size: 0.016713, time per step: 33.020 s, scan speed: 0.064274, and number of steps: 2633. The size and morphology of the nanoparticles were investigated by particle size analyzer make Malvern and model Zetasizer Nano and observed by scanning electron microscope model Tescan and make Vega 3 LMU. The nanoparticles were suspended in ethanol and deposited on a copper grid base, and allowed ethanol to evaporate. FTIR spectra of CA membrane and mixed matrix ZnO membranes were recorded by the Perkin Elmer spectrophotometer within the range of 450 to 4000 /cm.
Performance studies of membranes
In the current experiment, a lab scale membrane setup containing stainless steel disk membranes had been designed, fabricated (Fig. 6) and used to find the performance analysis of membranes with the effective membrane area in the module which is 28.26 cm2.
Pure water flux (PWF)
The PWF measurement was carried out in a batch mode and transmembrane pressure varying from 0.5 to 4 bar. The experiments were performed in an installation consisting of a feed tank, a pump, valve and two manometers. The system pressure and the flow are assured by the pump, and the pressure was adjusted by the valve. The flat plate permeation cells with two detachable parts were separated by a porous plate. PWF has been calculated as follows:
where J is the pure water flux (L/M2H1), V is the amount of permeate collected (L), Δt is the time interval of collection (H) and A is the membrane area (M2).
Hydraulic permeability
The hydraulic permeability of the membrane was obtained from the slope of the straight line between pure water flux and various applied transmembrane pressure. The equation is
where Lp is the hydraulic permeability (L/M2H1Bar1), J is the pure water flux (L/M2H1), and ΔP is the pressure (bar)
Analysis of arsenic
The concentration of Arsenic in water was analyzed using Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES), Model-Agilent ICP-OES 700 Series by set the wavelength (187, 197, 198 nm), using the concentration of known standards.
Solute rejection capacity calculation
The efficiency of rejection capability of CA and CA-ZnO nanoparticle mixed matrix membranes, the following rejection equation was used.
where R is the solute rejection, Cp is the solute concentration in permeate and Cf is that in the feed stream.
Results and discussion
Analysis of zinc oxide nanoparticle
Zinc oxide nano-sized particles XRD diffraction patterns are shown in Fig. 1. All the patterns of ZnO nanoparticles were observed as crystal characteristic peaks at 31.76°, 34.40°, 36.26°, 47.58°, 56.51°, 62.77°, 66.36°, 67.94°, 69.10°, 72.53°, and 76.87° corresponding to Chinese white and Zincite, respectively, known as hexagonal wurtzite structure [32, 40, 44]. The wurtzite structure is most stable at ambient conditions, a good hydrophilic agent which is suitable to modify the membrane.
Figure 2 shows the SEM images of the zinc oxide nanoparticles. The size of the nanoparticles varies from 50 to 500 nm. The result of ZnO using particle size analyzer is shown in Fig. 3. The Z-average particle size of the ZnO is equal to 198.3 nm.
Membrane morphology
To identify the presence of ZnO nanoparticles and the binding of the metal oxide nanoparticle to the polymer matrix were carried out by the FTIR [64]. FTIR spectrum for the membranes CA1, CA3 and CA5 and CA1-ZnO, CA3-ZnO and CA5-ZnO is shown in Fig. 4. A prominent and broad band at 3500-3200 assigned to O–H [60, 68], H-bonded, a strong band at 1742/cm was assigned to the carbonyl C=O stretching [59], band ranging from 3000 to 2850 assigned to C–H [59, 60], a week bond ranging from 2260 to 2100 corresponds to –C≡C– stretch. The bending fundamental (~OH) for the adsorbed water, which was located at 1636/cm. 1650–1580 assigned to the medium bond of N–H bend, a strong band at 1380–1370 was assigned to CH3 compound, 1280–1180 range was assigned to strong C–N stretch, 1320–1000 was assigned to the strong C–O stretch, 950–900 [64] the range was assigned to CH2 out of plane wag. However, the zinc-related band is not observed due to the presence of less quantity. SEM and EDAX were performed for the CA and CA–ZnO membranes shown in Fig. 5A and B, respectively (Fig. 6).
A SEM for CA and CA–ZnO membranes. Whereas a, c and e are representing CA1, CA3 and CA5; b, d and f are representing CA1–ZnO, CA3–ZnO and CA5–ZnO membranes. B EDAX for CA and CA-ZnO membranes. Whereas a, c and e are representing CA1, CA3 and CA5; b, d and f are representing CA1–ZnO, CA3–ZnO and CA5–ZnO membranes
Performance analysis of membranes
Effect of pressure on pure water flux and hydraulic permeability
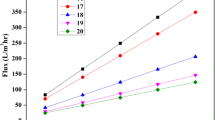
To determine the pure water flux permeation studies conducted for CA membranes and CA–ZnO membranes are shown in Tables 3, 4 and Figs. 7, 8 and Supplementary Figs. 1–4 and hydraulic permeability is shown in Table 5; deionized water is used for the study. The pure water flux was determined by “weighing the permeate volume (V) collected in a given time (t) and per membrane area (A) as J = V/(A × t)”. The hydraulic permeability of the membrane is obtained from “the slope of the straight line between pure water flux and various applied transmembrane pressure”. Transmembrane pressure is ranging from 0.5, 1, 1.5, 2, 3 and 4 bar. The pure flux values of CA membrane with 100 µm and 200 µm thickness were shown against various pressures applied. It has seen that the pure water flux of the membranes increases with increase in the pressure applied. As seen above, pure water flux decreases from CA1 to CA3 membranes and CA1–ZnO to CA5–ZnO. It can be attributed to the lower concentration of swelling agent formamide in CA5 membrane making it less porous. It shows that there was an increase of about 5–25% in the flux after adding ZnO nanoparticles. The presence of ZnO nanoparticles shows a positive increase in the hydrophilicity of the membrane making it more porous [11, 25, 32]. The hydraulic permeability of the membranes varied in the order CA1–ZnO > CA1 > CA3–ZnO > CA3 > CA5–ZnO > CA5 for both 100 µm and 200 µm thickness.
In filtration model to overcome the conflicts by the membranes, various pressures were applied to study the removal efficiency of the arsenic from the initial 1000 mg/L concentration solution. In general, flux is directly proportional to the pressure applied on the system to obtain the maximum arsenic removal without affecting the membrane. In this study, various pressures 0.5, 1, 2, 3 and 4 bars were applied to carry out the study. Till 2 bars of pressure the 100 µm CA membranes are able to sustain the pressure; beyond the 2 bar of pressure, the CA membranes were ruptured. However, the 200 µm CA and CA–ZnO membranes were able to sustain the pressures till 4 bar. Beyond the 4 bar pressure, the only CA–ZnO membranes are able to sustain till 5 bar showing that the incorporation of the ZnO nanoparticles increases the strength of the membranes. In this context, all the experiments are carried out at 1 bar of pressure to minimize the wear and tear of the CA membranes.
Effect of pH on removal of arsenic
To observe the effect of pH for the removal of arsenic using the cellulose acetate membranes with and without ZnO nanoparticles, the experiments are carried out ranging the pH from 3 to 9. The results showed the good removal efficiency at the neutral pH 7 only, because ZnO was amphoteric and it dissolves in acidic and basic pH and forms complex like (Zn(OH)4)2−. All the experiments are carried out at pH 7 ± 0.5.
Effect of ZnO nanoparticles on removal of arsenic
In the current experiment, different quantities of ZnO nanoparticles were incorporated onto the cellulose acetate membrane to carry out filtration studies for the removal of arsenic. Initially, the 1000 mg/l arsenic stock solution was prepared and used to carry out the arsenic removal studies with CA and CA–ZnO membranes. In CA–ZnO membranes, the ZnO nanoparticles range from 0, 0.25, 0.5 0.75, 1.0, 1.25, 1.5 g to obtain the final weight of 100 g of casting solution by adjusting the formamide and acetone weights. From the obtained results, it is found that 1 g of ZnO nanparticles incorporation onto the cellulose acetate (CA5–ZnO) membranes shows the 58.77% of arsenic removal.
Removal efficiency (RE) of arsenic
CA membranes prepared by phase inversion technique were used to study their performance in the removal of arsenic. Using cross flow filtration cell unit, the membrane rejection has been tested under operating pressure of 1 bar, pH 7 ± 0.5 and initial concentration of 1000 mg/L. The membranes were subjected to 4 h of filtration and permeate was collected at every 1 h and the removal efficiency is shown in Tables 6, 7 and Supplementary Tables 1–6.
Figure 9 and Table 6 show the relationship between the % removal efficiency of arsenic with respect to time for different cellulose acetate membranes. From the above figure, the removal efficiency of arsenic from water using CA1 membrane was found to 30 ± 1% for both 100 µm and 200 µm thickness membrane. The removal efficiency is slightly found to decline after 4 h. For CA3 membrane, the removal efficiency was found to be 33 ± 1% for 200 µm and 31 ± 1% for 100 µm thickness. The removal efficiency of arsenic from water using CA5 membrane for 100 µm thickness was found to be 37 ± 1% and for 200 µm, the removal efficiency was around 40 ± 1% until 2 h and then decreases to 38.75% at 4 h.
Table 7 and Fig. 10 show the removal efficiency of arsenic by cellulose acetate–ZnO nanoparticle mixed matrix membrane. For CA1–ZnO membrane, the removal efficiency is found be around 43 ± 1% for 100 µm and 47 ± 1% for 200 µm thickness membrane. As compared to CA1 membrane, CA1–ZnO shows higher arsenic removal efficiency. CA3–ZnO membranes arsenic removal efficiency was 48 ± 1% and 46 ± 1% for 200 µm and 100 µm, respectively. In the case of CA5–ZnO membranes, the removal efficiency was found to be around 57 ± 1% for 200 µm and for 100 µm thickness membrane 53 ± 1%. Removal efficiency follows the following trend, CA5–ZnO > CA3–ZnO > CA1–ZnO. Comparing CA membranes and CA–ZnO membranes, the removal efficiency of CA with ZnO nanoparticle membranes was increased.
Membrane charge and pore size play a vital role in separation of ionic species by nanofiltration membrane. The increase in the saturation time and removal efficiency from CA1 < CA3 < CA5 membrane was due to decrease in the pore size of the membrane and more straining due to the presence of lesser amount of formamide in the preparation of the CA5 membrane. The clogging of the microspaces available till the saturation time further retards the filtration process as explained above.
Conclusion
It can be observed that there was an increase of about 5% to 25% in the flux after adding ZnO nanoparticles. The presence of ZnO nanoparticles shows a positive increase in the hydrophilicity of the membrane making it more porous. As(III) was found to be in the form of the uncharged species H3AsO3 in natural waters and removal of As(III) is mainly attributed to steric exclusion than charge effects. Comparing CA membranes and CA–ZnO membranes, the removal efficiency of CA with ZnO nanoparticle membranes was increased.
References
Adak, A., Bandyopadhyay, M., Pal, A.: Removal of anionic surfactant from wastewater by alumina: a case study. Colloids Surf. A 254, 165–171 (2005)
Akin, I., Arslan, G., Tor, A., Cengeloglu, Y., Ersoz, M.: Removal of arsenate [As (V)] and arsenite [As (III)] from water by SWHR and BW-30 reverse osmosis. Desalination 281, 88–92 (2011)
Arora, M., Maheshwari, R., Jain, S., Gupta, A.: Use of membrane technology for potable water production. Desalination 170, 105–112 (2004)
Arsenic W (1981) environmental health criteria 18 Geneva: World Health Organization, p. 82
Arthanareeswaran, G., Devi, T.S., Raajenthiren, M.: Effect of silica particles on cellulose acetate blend ultrafiltration membranes: part I. Sep. Purif. Technol. 64, 38–47 (2008)
Baker, R.W.: Membrane technology. Wiley Online Library (2000). https://doi.org/10.1002/0471238961.1305130202011105.a01
Bottino, A., Capannelli, G., D’asti, V., Piaggio, P.: Preparation and properties of novel organic–inorganic porous membranes. Sep. Purif. Technol. 22, 269–275 (2001)
Chatterjee, A., Das, D., Mandal, B.K., Chowdhury, T.R., Samanta, G., Chakraborti, D.: Arsenic in ground water in six districts of West Bengal, India: the biggest arsenic calamity in the world. I: arsenic species in drinking water and urine of the affected people. Analyst 120, 643–650 (1995)
Choong, T.S., Chuah, T., Robiah, Y., Koay, F.G., Azni, I.: Arsenic toxicity, health hazards and removal techniques from water: an overview. Desalination 217, 139–166 (2007)
Chowdhury, T.R., et al.: Arsenic poisoning in the Ganges delta Nature 401, 545–546 (1999)
Chung, Y.T., Ba-Abbad, M.M., Mohammad, A.W., Benamor, A.: Functionalization of zinc oxide (ZnO) nanoparticles and its effects on polysulfone-ZnO membranes. Desalin. Water Treat. 57, 7801–7811 (2016). https://doi.org/10.1080/19443994.2015.1067168
Cutter, G.A.: Kinetic controls on metalloid speciation in seawater. Mar. Chem. 40, 65–80 (1992)
Del Razo, L., Arellano, M., Cebrián, M.E.: The oxidation states of arsenic in well-water from a chronic arsenicism area of northern Mexico. Environ. Pollut. 64, 143–153 (1990)
Dixit, S., Hering, J.G.: Comparison of arsenic (V) and arsenic (III) sorption onto iron oxide minerals: implications for arsenic mobility. Environ. Sci. Technol. 37, 4182–4189 (2003)
Ebert, K., Fritsch, D., Koll, J., Tjahjawiguna, C.: Influence of inorganic fillers on the compaction behaviour of porous polymer based membranes. J. Membr. Sci. 233, 71–78 (2004)
Edmunds, W.M., Cook, J.M., Kinniburgh, D.G., Miles, D.L., Trafford, J.M.: Trace–element occurrence in British groundwaters, British Geological Survey. Report SD/89/3 Keyworth, Nottingham, UK. 424 (1989)
Figueiredo, A.S., Sánchez-Loredo, M.G., Maurício, A., Pereira, M.F., Minhalma, M., de Pinho, M.N.: Tailoring of structures and permeation properties of asymmetric nanocomposite cellulose acetate/silver membranes. J. Appl. Polym. Sci. 132, 1–11 (2015)
Fogarassy, E., Galambos, I., Bekassy-Molnar, E., Vatai, G.: Treatment of high arsenic content wastewater by membrane filtration. Desalination 240, 270–273 (2009)
Goh, P.S., Ismail, A.F., Sanip, S.M., Ng, B.C., Aziz, M.: Recent advances of inorganic fillers in mixed matrix membrane for gas separation. Sep. Purif. Technol. 81, 243–264 (2011)
Gopal, R., Kaur, S., Ma, Z., Chan, C., Ramakrishna, S., Matsuura, T.: Electrospun nanofibrous filtration membrane. J. Membr. Sci. 281, 581–586 (2006)
Hajipour, M.J., et al.: Antibacterial properties of nanoparticles. Trends Biotechnol 30, 499–511 (2012)
Han, B., Runnells, T., Zimbron, J., Wickramasinghe, R.: Arsenic removal from drinking water by flocculation and microfiltration. Desalination 145, 293–298 (2002)
Ho, W.S.W., Sirkar, K.K.: Handbook M. Von Nostrand Reinhold, New York (1992)
Jain, C., Ali, I.: Arsenic: occurrence, toxicity and speciation techniques. Water Res. 34, 4304–4312 (2000)
Jo, Y.J., Choi, E.Y., Choi, N.W., Kim, C.K.: Antibacterial and hydrophilic characteristics of poly(ether sulfone) composite membranes containing zinc oxide nanoparticles grafted with hydrophilic polymers. Ind. Eng. Chem. Res. 55, 7801–7809 (2016). https://doi.org/10.1021/acs.iecr.6b01510
Joshi, A., Chaudhuri, M.: Removal of arsenic from ground water by iron oxide-coated sand. J. Environ. Eng. 122, 769–771 (1996)
Kim, J., Van der Bruggen, B.: The use of nanoparticles in polymeric and ceramic membrane structures: review of manufacturing procedures and performance improvement for water treatment. Environ. Pollut. 158, 2335–2349 (2010)
Kolodziejczak-Radzimska, A., Jesionowski, T.: Zinc oxide-from synthesis to application: A review. Materials 7, 2833–2881 (2014). https://doi.org/10.3390/ma7042833
Koros, W., Fleming, G.: Membrane-based gas separation. J. Membr. Sci. 83, 1–80 (1993)
Koros, W.J., Mahajan, R.: Pushing the limits on possibilities for large scale gas separation: which strategies? J. Membr. Sci. 175, 181–196 (2000)
Korte, N.E., Fernando, Q.: A review of arsenic (III) in groundwater. Crit. Rev. Environ. Sci. Technol. 21, 1–39 (1991)
Kumar, S.S., Venkateswarlu, P., Rao, V.R., Rao, G.N.: Synthesis, characterization and optical properties of zinc oxide nanoparticles. Int. Nano Lett. 3, 30 (2013)
Kunst, B., Sourirajan, S.: An approach to the development of cellulose acetate ultrafiltration membranes. J. Appl. Polym. Sci. 18, 3423–3434 (1974)
Lepkowski, W.: Arsenic crisis in Bangladesh. Chem. Eng. News 76, 27–29 (1998)
Mandal, B.K., Suzuki, K.T.: Arsenic round the world: a review. Talanta 58, 201–235 (2002)
Manimegalai, G., Kumar, S.S., Sharma, C.: Pesticide mineralization in water using silver nanoparticles. Int. J. Chem. Sci. 9, 1463–1471 (2011)
Manimegalai, G., Shanthakumar, S., Sharma, C.: Silver nanoparticles: synthesis and application in mineralization of pesticides using membrane support. Int. Nano Lett. 4, 105 (2014)
McArthur, J., Ravenscroft, P., Safiulla, S., Thirlwall, M.: Arsenic in groundwater: testing pollution mechanisms for sedimentary aquifers in Bangladesh. Water Resour. Res. 37, 109–117 (2001)
McArthur, J.M.: Reply: arsenic poisoning in the Ganges delta. Nature 401, 546–547 (1999)
Moballegh, A., Shahverdi, H.R., Aghababazadeh, R., Mirhabibi, A.R.: ZnO nanoparticles obtained by mechanochemical technique and the optical properties. Surf. Sci. 601, 2850–2854 (2007). https://doi.org/10.1016/j.susc.2006.12.012
Mohammad, A.W., Teow, Y.H., Ang, W.L., Chung, Y.T., Oatley-Radcliffe, D.L., Hilal, N.: Nanofiltration membranes review: recent advances and future prospects. Desalination 356, 226–254 (2015)
Mohan, A.C., Renjanadevi, B.: Preparation of zinc oxide nanoparticles and its characterization using scanning electron microscopy (SEM) and X-ray diffraction (XRD). Procedia Technol. 24, 761–766 (2016)
Mondal, S., Chatterjee, S., De, S.: Theoretical investigation of cross flow ultrafiltration by mixed matrix membrane: a case study on fluoride removal. Desalination 365, 347–354 (2015)
Mubarak, T.H., Hassan, K.H., Abbas, Z.M.A.: Using X-ray diffraction and scanning electron microscope to study zinc oxide nanoparticles prepared by wet chemical method. Adv. Mater. Res. (2013). https://doi.org/10.4028/www.scientific.net/AMR.685.119. (Dubai)
Ng, L.Y., Mohammad, A.W., Leo, C.P., Hilal, N.: Polymeric membranes incorporated with metal/metal oxide nanoparticles: a comprehensive review. Desalination 308, 15–33 (2013)
Nguyen, V., Vigneswaran, S., Ngo, H., Shon, H., Kandasamy, J.: Arsenic removal by a membrane hybrid filtration system. Desalination 236, 363–369 (2009)
Nickson, R., McArthur, J., Burgess, W., Ahmed, K.M., Ravenscroft, P., Rahmanñ, M.: Arsenic poisoning of Bangladesh groundwater. Nature 395, 338 (1998)
Nicolaisen, B.: Developments in membrane technology for water treatment. Desalination 153, 355–360 (2003)
Noble, R.D., Stern, S.A.: Membrane separations technology: principles and applications, vol. 2. Elsevier, New York (1995)
Purkait, M., DasGupta, S., De, S.: Removal of dye from wastewater using micellar-enhanced ultrafiltration and recovery of surfactant. Sep. Purif. Technol. 37, 81–92 (2004)
Raghupathi, K.R., Koodali, R.T., Manna, A.C.: Size-dependent bacterial growth inhibition and mechanism of antibacterial activity of zinc oxide nanoparticles. Langmuir 27, 4020–4028 (2011)
Rosa, M.J., de Pinho, M.N.: Separation of organic solutes by membrane pressure-driven processes. J. Membr. Sci. 89, 235–243 (1994)
Saranya, R., Arthanareeswaran, G., Ismail, A., Dionysiou, D.D., Paul, D.: Zero-valent iron impregnated cellulose acetate mixed matrix membranes for the treatment of textile industry effluent RSC. Advances 5, 62486–62497 (2015)
Schwarzenbach, R.P., Escher, B.I., Fenner, K., Hofstetter, T.B., Johnson, C.A., Von Gunten, U., Wehrli, B.: The challenge of micropollutants in aquatic systems. Science 313, 1072–1077 (2006)
Sen, M., Manna, A., Pal, P.: Removal of arsenic from contaminated groundwater by membrane-integrated hybrid treatment system. J. Membr. Sci. 354, 108–113 (2010)
Sharma, D., Rajput, J., Kaith, B., Kaur, M., Sharma, S.: Synthesis of ZnO nanoparticles and study of their antibacterial and antifungal properties. Thin Solid Films 519, 1224–1229 (2010)
Shih, M.-C.: An overview of arsenic removal by pressure-drivenmembrane processes. Desalination 172, 85–97 (2005)
Singh, R.: Hybrid membrane systems for water purification: technology, systems design and operations. Elsevier (2006)
Sonawane, S.H., Terrien, A., Figueiredo, A.S., Clara Goncalves, M., De Pinho, M.N.: The role of silver nanoparticles on mixed matrix Ag/cellulose acetate asymmetric membranes. Polym. Compos. (2015). https://doi.org/10.1002/pc.23557
Thirumavalavan, M., Huang, K.-L., Lee, J.-F.: Preparation and morphology studies of nano zinc oxide obtained using native and modified chitosans. Materials 6, 4198–4212 (2013)
Vatanpour, V., Madaeni, S.S., Khataee, A.R., Salehi, E., Zinadini, S., Monfared, H.A.: TiO2 embedded mixed matrix PES nanocomposite membranes: influence of different sizes and types of nanoparticles on antifouling and performance. Desalination 292, 19–29 (2012)
Welch, A.H., Westjohn, D., Helsel, D.R., Wanty, R.B.: Arsenic in ground water of the United States: occurrence and geochemistry. Groundwater 38, 589–604 (2000)
Wickramasinghe, S., Han, B., Zimbron, J., Shen, Z., Karim, M.: Arsenic removal by coagulation and filtration: comparison of groundwaters from the United States and Bangladesh. Desalination 169, 231–244 (2004)
Wilson, E.B., Decius, J.C., Cross, P.C.: Molecular vibrations: the theory of infrared and Raman vibrational spectra. Courier Corporation, Chelmsford (1955)
Yan, L., Li, Y.S., Xiang, C.B.: Preparation of poly (vinylidene fluoride)(pvdf) ultrafiltration membrane modified by nano-sized alumina (Al2O3) and its antifouling research. Polymer 46, 7701–7706 (2005)
Yan, L., Li, Y.S., Xiang, C.B., Xianda, S.: Effect of nano-sized Al2O3-particle addition on PVDF ultrafiltration membrane performance. J. Membr. Sci. 276, 162–167 (2006)
Yang, Y., Zhang, H., Wang, P., Zheng, Q., Li, J.: The influence of nano-sized TiO2 fillers on the morphologies and properties of PSF UF membrane. J. Membr. Sci. 288, 231–238 (2007)
Zak, A.K., Majid, W.A., Darroudi, M., Yousefi, R.: Synthesis and characterization of ZnO nanoparticles prepared in gelatin media. Mater. Lett. 65, 70–73 (2011)
Zimmerman, CM.: Advanced gas separation membrane materials: hyper rigid polymers and molecular sieve-polymer mixed matrices. PhD diss., University of Texas at Austin (1998)
Zimmerman, C.M., Singh, A., Koros, W.J.: Tailoring mixed matrix composite membranes for gas separations. J. Membr. Sci. 137, 145–154 (1997)
Acknowledgements
The authors thank the Council of Scientific and Industrial Research (CSIR) EMR-II, Govt. of India for the sponsorship of the project #02(0215)/14/EMR-II sanctioned to Dr. R Satish Babu, Dr. Anand Kishore Kola and Dr. Shirish H. Sonawane.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Potla Durthi, C., Rajulapati, S.B., Palliparambi, A.A. et al. Studies on removal of arsenic using cellulose acetate–zinc oxide nanoparticle mixed matrix membrane. Int Nano Lett 8, 201–211 (2018). https://doi.org/10.1007/s40089-018-0245-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40089-018-0245-3