Abstract
The present study demonstrates the bioreductive green synthesis of nanosized HgO using flower extracts of an ornamental plant Callistemon viminalis. The flower extracts of Callistemon viminalis seem to be environmentally friendly, so this protocol could be used for rapid production of HgO. Till date, there is no report of synthesis of nanoparticles using flower extract of Callistemon viminalis. Mercuric acetate was taken as the metal precursor in the present experiment. The flower extract was found to act as a reducing as well as a stabilizing agent. The phytochemicals present in the flower extract act as reducing agent which include proteins, saponins, phenolic compounds, phytosterols, and flavonoids. FT-IR spectroscopy confirmed that the extract had the ability to act as a reducing agent and stabilizer for HgO nanoparticles. The formation of the plant protein-coated HgO nanoparticles was first monitored using UV–Vis absorption spectroscopy. The UV–Vis spectroscopy revealed the formation of HgO nanoparticles by exhibiting the typical surface plasmon absorption maxima at 243 nm. The average particle size formed ranges from 2 to 4 nm. The dried form of synthesized nanoparticles was further characterized using TGA, XRD, TEM, and FTIR spectroscopy. FT-IR spectra of synthesized HgO nanoparticles were performed to identify the possible bio-molecules responsible for capping and stabilization of nanoparticles, which confirm the formation of plant protein-coated HgO nanoparticles that is further corroborated by TGA study. The optical band gap of HgO nanoparticle was measured to be 2.48 eV using cutoff wavelength which indicates that HgO nanoparticles can be used in metal oxide semiconductor-based photovoltaic cells. A possible core–shell structure of the HgO nanobiocomposite has been proposed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The green method of synthesis of nanoparticles is easy, efficient, and eco-friendly in comparison to chemically mediated synthesis [1–3]. The chemical synthesis involves toxic solvents, high pressure, energy, and high temperature conversion as well as microbe-involved synthesis which are not feasible industrially. Green synthesis is the best option to opt for the synthesis of nanoparticles, as it does not involve such a process. The HgO nanoparticles are synthesized using an aqueous extract of Callistemon viminalis and mercuric ions. Mercuric oxide is solid at room temperature and pressure. It occurs in two forms, red and yellow. The yellow form is a cyclodesulfarizing agent. Mercuric oxide is used in mercury batteries and as an efficient power source in space exploration. HgO is applied to the cathode in the mercury battery cell, which is widely used in small electronic equipments. It is also used in alkaline batteries and pigments, as seed protectant and as a preservative in cosmetics. It is used as a tropical antiseptic and in ointment form for eye disorders. Among the semiconductor compounds, HgO was suggested to be the most unusual in terms of its structural properties at ambient pressure, which are largely determined by the strong tendency for linear coordination of Hg to form the O–Hg–O chain.
Callistemon viminalis is a flowering plant of the family Myrtaceae. The plant is commonly called bottle brush due to the resemblance of its flower to the bristles of a bottle brush [1]. It is an evergreen tree having dense, multi-trunked, low-branching, pendulous growth habit and a moderate growth rate. Callistemon viminalis can reach up to 25–30 feet tall in 30 years, but most trees are 15–20 feet tall. They bloom in great abundance from March to July and less so throughout the year. Apart from its ornamental use in gardens for its aesthetic beauty, it has great medicinal importance. This plant has been used to treat haemorrhoids in China from ancient times. It is also a relaxant (antispasmodic activity) and has antithrombotic and repellent effect. Further, the plant has other useful properties which include nematocidal, larvicidal, pupicidal, insecticidal, antibacterial and antifungal properties [4, 5]. Callistemon viminalis is also used for weed control, as bioindicator for environmental management, and is a source of an essential oil [6].
The formation of nanoparticles is thermodynamically forbidden, as new surfaces are created which demands positive free energy of formation. Even after the formation of nanoparticles, to reduce the surface area nanoparticles tend to agglomerate resulting in sedimentation. Therefore, stabilization of nanoparticles is an important issue to be dealt with. In most of the cases, a separate stabilizing agent is required to avoid the agglomeration of nanoparticles.
The synthesized nanoparticles were characterized by UV spectroscopy, TEM spectroscopy, FTIR spectroscopy, X-ray spectroscopy and TGA analysis, which confirmed the formation of plant protein-coated HgO nanoparticles. The protein present in the flower extract acts as a reducing agent and stabilizes the formed nanoparticle by modifying its surface as a capping agent.
This is the very first report of the synthesis of plant protein-coated HgO nanoparticle synthesis using Callistemon viminalis flower extracts. This opens avenues for the convenient green synthesis of nanoparticles using possible biomolecules present in plants, which act as a reducing as well as stabilizing agent.
Materials and methods
Preparation of aqueous flower extract of Callistemon viminalis
The Callistemon viminalis flower material was collected from a location of latitude: 27 N 80′ 27.58 and longitude 75E 03′ 48.79″ (Guru Vashisht College, FASC, Lakshmangarh, Sikar District of Rajasthan Province of India). The flowers were dried and later finely powdered for extraction of phytochemicals present in them.
Fresh flowers of Callistemon viminalis were washed under running tap water to remove any attached debris and dust and subsequently with Millipore water three to four times [7]. Flowers were air dried for 2 weeks at room temperature (25 °C). The dried flowers were finely powdered by grinding using a Lumix grinder (AICIL, Chandigarh, India). The extract was prepared by taking 40 g of powdered flowers in a 500 mL round flask with 300 mL of sterile deionized distilled water (Millipore India Pvt Ltd.). The extract was further boiled for 10 min and sieved and filtered twice by using filter paper No. 42 (Whatman, USA). The filtrate was collected and stored at 4 °C and used within a week. A small amount of filtrate was dried at 80 °C and analysed by FT-IR techniques [8].
Phytochemical analysis
Phytochemical analysis of the Callistemon viminalis flower extract was carried out following the standard method available in the literature [9–11].
Tannins On adding 2 ml filtrate + 2 ml FeCl3, a blue-black precipitate indicated the presence of tannins.
Saponins Frothing test: on adding 0.5 ml filtrate + 5 ml distilled water, frothing persistence indicated the presence of saponins.
Glycosides On adding 2 ml filtrate + 1 ml glacial acetic acid + FeCl3 + conc. H2SO4, a green-blue colour indicated the presence of cardiac glycosides.
Phenolics 1 ml each of the concentrated extracts was heated to remove the solvent and the residues were taken in a small amount of aqueous methanol. To the methanolic solution, 0.5 % ferric chloride solution was added and the change in colour was marked in the alcoholic extract, indicating the presence of phenolic compounds.
Flavonoids To the test solution, a few drops of sodium hydroxide solution were added. An intense yellow colour was formed which turned colourless on adding a few drops of dilute acid, indicating the presence of flavonoids.
Alkaloids about 1 ml each of concentrated extracts was evaporated to dryness at a controlled temperature and then the residue was treated with 5 % hydrochloric acid (2 ml) and filtered. The filtrates were tested with Mayer’s and Dragendorff’s reagent. A creamish precipitate/brownish-red precipitate/orange precipitate indicated the presence of the respective alkaloids.
Sugars 15 ml of Fehling-“A” was mixed with 15 ml of Fehling -“B”. 2 ml of this mixture was added to an empty test tube. Three drops of the compound was taken to be tested in a tube, which was placed in a water bath at 60 °C. A positive test was indicated by a green suspension or a red precipitate.
Proteins 1 ml of the extract to be tested was taken in a test tube and five drops of conc. HNO3 added. It was placed in a hot water bath for half a minute. A yellow acid xanthoprotein substance was formed by the action of hot nitric acid on albuminous or protein matter that changed to a deep orange-yellow colour by the addition of ten drops of conc. ammonia or NaOH.
Starch On adding 1 ml of extract to the test tube + few drops of KI solution, a blue-black colour indicates the presence of starch.
Phytosterols To 1 ml of flower extract, conc. H2SO4 was added along the side of the test tube. A reddish brown interface form indicates the presence of steroids.
Antimicrobial activity
The antimicrobial susceptibility of plant protein-coated HgO nanoparticles was evaluated by keeping the plant crude extract and pure uncoated HgO nanoparticles as control using the disc diffusion method [9]. Penicillin discs were used as a positive indicator. The Petri dish was then subjected to an incubation at 37 °C overnight to allow the bacteria to grow [12]. The antimicrobial activity of plant protein-coated HgO nanoparticles was evaluated for Escherichia coli (Gram-negative bacteria; MTCC No 1687). The strain was obtained from Microbial Type Culture Collection depository of the Institute of Microbial Technology, Chandigarh, India [13].
Nanoparticles synthesis
Mercuric acetate was purchased from CDH, and the aqueous flower extract of Callistemon viminalis was used as a bioreductive agent. To synthesize nanoparticles from Callistemon viminalis, 40 mL of the aqueous flower extract was added to 100 mL of 4 mM aqueous mercuric acetate solution in 250 mL Schott Duran beaker. The beaker was subjected to an rpm of 150 at 60 °C.
Characterization of nanoparticles
Mercuric oxide (HgO) nanoparticles synthesized by this green method were initially examined using Carry 60 Agilent UV–Vis spectrophotometer. FT-IR spectroscopy of plant protein-coated mercuric oxide nanoparticles was carried in the range 4000 to 400 cm−1 using Perkin Elmer FT-IR spectrophotometer and compared with that of pure uncoated bulk HgO, confirming the protein coating on HgO nanoparticles which acts as a stabilizer for these. X-ray diffraction and TEM images were also taken to identify the nature of crystal and particle size, respectively. Thermogravimetric analysis (TGA) was performed under a nitrogen atmosphere at a heating rate of 10 °C/min from room temperature up to 700 °C, which further confirmed the formation of plant protein-coated HgO nanoparticles. The optical band gap of the nanocrystalline samples was calculated from the absorption peak as 2.48 eV.
Results and discussion
Bioreductive and green-synthesized mercuric oxide (HgO) nanoparticles were produced by treating mercuric ions with the flower extracts of an ornamental plant Callistemon viminalis. Mercuric acetate was taken as the metal precursor in the present experiment where the flower extract was found to act as a reducing as well as a stabilizing agent. The colour change during the formation of nanoparticles was visualized. The colour of the mercuric acetate/flower extract solution changed from light maroon to light brown after 5 min and eventually to dark brown. This colour change indicates the formation of HgO nanoparticles in solution. Mercuric acetate without flower extract did not show any colour change.
Ten different tests to identify phytochemicals in the aqueous extract of the ornamental plant Callistemon viminalis were conducted. Seven phytochemicals were identified by the tests which have been shown in Table 1. The phytochemicals present in the flower extract which act as reducing agents are tannins, saponins, phenolic compounds, flavonoids, sugars, proteins and steroids. The aqueous Callistemon viminalis flower extract was found to have these contents, suggesting that it was the most favourable starting material for the preparation of HgO nanoparticles.
The presence of protein in the plant extract has been confirmed by TLC study of the extract. The plate was developed with a stationary phase of silica gel on aluminium paper and the extract was charged and moved under the influence of the mobile phase of chloroform and methanol in 9:1 ratio for 4 h. Thereafter, ninhydrin was spread as a spraying reagent (prepared by taking 0.2 gm of ninhydrin in 100 ml ethanol), followed by heating at 110 °C till a reddish spot appeared in the plate which confirmed the presence of protein in the extract. It was later corroborated by FTIR and TGA studies (Fig. 1).
Natural alternative treatments for bacterial infections may provide a pathway for the development of new antimicrobial agents. The antimicrobial activity of the aqueous Callistemon viminalis flower extracts-mediated plant protein-coated HgO nanoparticles was investigated using E. coli (Fig. 2) along with pure uncoated HgO and crude plant extract. Standard penicillin was taken as a positive control. The results showed that the antimicrobial potential of plant protein-coated HgO nanoparticles still prevailed, as it was present in the pure uncoated bulk HgO. The crude plant extract did not have any antibacterial activity.
The formation of HgO nanoparticles was first confirmed by using UV–visible spectroscopy (UV–Vis). No other peak was observed in the spectrum, confirming that the synthesized product was HgO only. Generally, biosynthetic methods are considered as time consuming when compared with chemical methods. To the best of our knowledge, the reaction time for the formation of plant protein-mediated HgO nonmaterial is 15 min.
The UV–Vis spectroscopy revealed the formation of HgO nanoparticles by exhibiting the typical surface plasmon absorption maxima at 243 nm (Fig. 3). The surface plasmon resonance of the pure uncoated bulk HgO was earlier reported by Mukherjee et al. [14]. A comparative study of UV–Vis spectra of flower extract, mercuric acetate solution and freshly prepared plant protein-coated HgO nanoprticles has been depicted to confirm the formation of pure plant protein-coated HgO nanoparticles.
a UV–visible spectrum of Callistemon viminalis flower extract. b UV–visible spectrum of 4 mM mercuric acetate solution before treatment with flower extracts. c UV–visible spectrum of mercuric acetate solution after treatment with flower extracts. The characteristic peak around 243 nm was obtained, confirming the synthesis of HgO nanoparticles
The optical band gap of the nanocrystalline samples was calculated from the absorption peak using the formula:
H = Planck’s constant = 6.626 × 10−34 J s, c = Speed of light = 3.0 × 108 m/s, λ = Cutoff wavelength (nm) where the absorbance value is minimum = 390 nm (Fig. 5c),
where 1 eV = 1.6 × 10−19 J (conversion factor),
The colloidal solutions were dried in watch glasses to analyse the samples. The dried form of synthesized plant protein-coated HgO nanoparticles was further characterized using Fourier transform infrared (FTIR) spectroscopy. The FTIR data were compared with pure uncoated bulk HgO [15] as mentioned in the inset of Fig. 4 to identify the likely biomolecules accountable for capping and reducing of the HgO nanoparticles produced from Callistemon viminalis.
The synthesized plant protein-coated HgO nanoparticles showed 11 infrared bands at 3432, 1640, 1573, 1414, 1384, 1074, 1021, 928, 865, 781 and 650 cm−1 (Fig. 4). The peaks at 1414 and 1021 cm−1 are attributed to the asymmetric and symmetric stretching vibration of COO− that proves the combination of the protein with the nanoparticles. The peak at 1640 cm−1 is responsible for the presence of the amide carbonyl group of protein, which is attached to the synthesized nanoparticle as a capping and stabilizing agent.
The IR spectrum of HgO nanoparticles shows that the absorption peaks at 650 and 471 cm−1 are assigned to the (Hg–O) mode, confirming the formation of HgO nanoparticles which is further corroborated by FTIR of pure uncoated bulk HgO data mentioned in the inset of Fig. 4 [15].
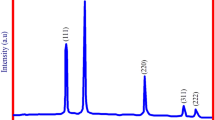
XRD is an effective characterization to confirm the crystal structure of the synthesized mercuric oxide nanoparticles. Ten characteristic peaks at 16.4, 22.7, 28.4, 31.8, 34.3, 35.1, 37.7, 48.3 and 54.4 corresponded to the (100), (111), (200), (210), (211), (211), (220), (221), (311) and (321) crystal planes of a pure HgO. They matched well with that of JCPDS No. 37–1469, indicating that the sample has a cubic crystal system. No characteristic peak for impurity was observed (Fig. 5).
The transmission electron microscopy (TEM) micrograph for synthesized mercuric oxide nanoparticles is shown in Fig. 6. The particles had a rather narrow size distribution, where most of the mercuric oxide particles were within 2–4 nm. Therefore, the HgO nanoparticles were successfully synthesized by this green method using Callistemon viminalis leaf extract as reducing agent and stabilizer for nanoparticles.
Figure 7 revealed the TGA results for these HgO nanoparticles. An initial weight loss of 6.11 % around 120 °C occurred, followed by 9.66, 2.36 and 5.45 % at 340, 510 and 699 °C, respectively, thus confirming that the plant protein was conjugated to the HgO nanoparticles forming the nanoparticles–plant protein hybrid. The initial weight loss of HgO nanoparticles powder under 100 °C is likely to be caused by the water contained in it.
The protein of Callistemon viminalis leaf extract decomposes completely at temperatures higher than 600 °C and the residual weight of HgO nanoparticles is 76.39 % at 700 °C. The results of TGA illustrated that amide I and amide II were present in Callistemon viminalis leaf extract in the HgO nanoparticles with a weight of around 34 %. TGA demonstrated that Callistemon viminalis leaf extract existed on the surface of HgO nanoparticles.
The FTIR and TGA study of the green-synthesized HgO nanoparticles confirms the protein coating on the nanoparticle surface which gives stabilization to the nanoparticles, preventing agglomeration. Based on the study, a possible core–shell structure of the HgO nanobiocomposite has been proposed in Fig. 8.
Conclusion
In the present study, we report a green approach for the synthesis of HgO nanoparticles using flower extracts of Callistemon viminalis. The flower extracts of Callistemon viminalis seem to be environmentally friendly, so that this protocol can be used for the rapid production of HgO. Till date, there has been no report of the synthesis of plant protein-coated nanoparticles forming core–shell structure of the HgO nanobiocomposite using flower extract of Callistemon viminalis. Investigation of the antibacterial activity of HgO nanoparticles against E. coli reveals the high potential of Callistemon viminalis flower extract-stabilized HgO nanoparticles for use as antimicrobial agent in the medical field as well as in food, medicine and cosmetic industries. The optical band gap has been calculated to be below 3 eV and the plant protein-coated HgO nanoparticles show a promise to be used in metal oxide semiconductor-sensitized photovoltaic cell.
Authors contribution
All the authors contributed equally.
References
Yallapu, M.M., Foy, S.P., Jain, T.K., Labhasetwar, V.: PEG-Functionalized Magnetic Nanoparticles for Drug Delivery and Magnetic Resonance Imaging Applications. Pharm. Res. 27, 2283–2295 (2010)
Mahdavi, M., Namvar, F., Ahmad, M.B., Mohamad, R.: Molecules 18, 5954–5964 (2013)
Pattanayak, M., Nayak, P.L.: Int J Plant 3, 68–78 (2013)
Shinde, P.R., Patil, P.S., Bairagi, V.A.: Pharmacognostic, phytochemical properties and antibacterial activity of Callistemon citrinus viminalis leaves and stems. Int J Pharm Pharmaceut Sci 4, 406–408 (2012)
Oyedeji, O.O., Lawal, O.A., Shode, F.O., Oyedeji, A.O.: Chemical Composition and Antibacterial Activity of the Essential Oils of Callistemon citrinus and Callistemon viminalis from South Africa. Molecules 14, 1990–1998 (2009)
Delahaye, C., Rainford, L., Nicholson, A., Mitchell, S., Lindo, J., Ahmad, M.: Antibacterial and antifungal analysis of crude extracts from the leaves of Callistemon viminalis. J Med Biol Sci 3, 1–7 (2009)
Ghosh, S., Patil, S., Ahire, M., Kitture, R., Gurav, D.D., Jabgunde, A.M., Kale, S., Pardesi, K., Shinde, V., Bellare, J., Dhavale, D.D., Chopade, B.A.: Gnidia glauca flower extract mediated synthesis of gold nanoparticles and evaluation of its chemocatalytic potential. J Nanobiotech 10, 17 (2012)
Kumar, P., Singh, P., Kumari, K., Mozumdar, S., Chandra, R.: A green approach for the synthesis of gold nanotriangles using aqueous leaf extract of Callistemon viminalis. Mater. Lett. 65, 595–597 (2011)
Parekh, J., Chanda, S.V.: In vitro Antimicrobial Activity and Phytochemical Analysis of Some Indian Medicinal Plants. Turk J Biol. 31, 53–58 (2007)
Prabhu, K., Karar, P.K., Hemalatha, S., Ponnudurai, K.: A comparative preliminary phytochemical screening on the leaves, stems and the roots of three viburnum Linn Species. Der Pharmacia Sinica. 2, 81–93 (2013)
Arunachalam, K.D., Annamalai, S.K., Hari, S.: One-step green synthesis and characterization of leaf extract-mediated biocompatible silver and gold nanoparticles from Memecylon umbellatum. Int J Nanomed 8, 1307–1315 (2013)
Guzmán, M.G., Dille, J., Godet, S.: Synthesis of silver nanoparticles by chemical reduction method and their antibacterial activity. Int J Chem Biol Eng 2, 3 (2009)
Ramteke, C., Chakrabarti, T., Sarangi, B.K., Pandey, R.A.: Synthesis of silver nanoparticles from the Aqueous extract of leaves of Ocimum sanctum for enhanced antibacterial activity. J Chem 278925, 7 (2013)
Mukherjee, I., Senapati, S., Mitra, D., Rakshit, A.K., Das, A.R., Moulik, S.P.: Physiochemistry of dispersion of HgO, HgS and Makardhwaj (an Ayurvedic medicine) prepared in micelle and microemulsion templates, Colloids and Surfaces A: Physicochem. Eng. Aspects. 360, 142–149 (2010)
Askarinejad, A., Morsali, A.: Synthesis and characterization of mercury oxide unusual nanostructures by ultrasonic method. Chem. Eng. J. 153, 183–186 (2009)
Acknowledgments
Thanks are due to the Department of Atomic Energy (DAE), Board of Research in Nuclear Sciences (BRNS), Mumbai, India, for the financial support. The help and support of Dr. R K Gaur, Mr. Rakesh Kumar Verma and Mr. Ritesh Mishra of FASC, Mody University are highly acknowledged.
Conflict of interest
The content and authorship of this manuscript have been approved by all the authors and all prevailing local, national and international regulations and conventions, and normal scientific ethical practices, have been respected. The authors have no conflict of interest to declare including any financial, personal or other relationships with other people or organizations. With the submission of this manuscript we would like to undertake that the above-mentioned manuscript has not been published elsewhere, accepted for publication elsewhere or under editorial review for publication elsewhere, and that our Institute’s Mody University of Science and Technology representative is fully aware of this submission.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Das, A.K., Marwal, A., Sain, D. et al. One-step green synthesis and characterization of plant protein-coated mercuric oxide (HgO) nanoparticles: antimicrobial studies. Int Nano Lett 5, 125–132 (2015). https://doi.org/10.1007/s40089-015-0144-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40089-015-0144-9