Abstract
The potential applications of one-dimensional nanostructured materials widely depend on their shape and size. This paper deals with a method for synthesizing and characterizing silver nanowires through an organic solution. The so-called polyol method was used to synthesize silver nanowires in which ethylene glycol was used as both media and the reducing agent. Adequate CuCl2/ethylene glycol solution is used to prohibit chemical etching by the oxygen on nanowires surfaces. The effects of CuCl2 concentration and the addition rate of AgNO3 were examined. It was revealed that by optimizing the parameters, silver nanowires with aspect ratios of up to 32 are accessible. To characterize the structure and morphology of nanowires, X-ray powder diffraction, UV–Vis spectroscopy, scanning electron microscopy and atomic force microscopy were used.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Presenting a straightforward method for synthesizing nanostructured materials is becoming a hot area of research because of their potential applications in optic, electronic and magnetic devices [1–5]. Among all 1D nanostructures, a great deal of effort has been made to synthesize silver nanowires due to their highest electrical and thermal conductivity among all metals [6]. Using chemical methods, various routes have been used for synthesizing silver nanowires.
Xia et al. [7] demonstrated a large-scale method for synthesizing silver nanowires in which ethylene glycol (EG) acts as both solvent and the reducing agent. Polyvinylpyrrolidone (PVP) acts as a capping agent that blocks the {100} planes of the formed multiply-twinned particles (MTPs), thus helping the formation of silver nanowires. However, in such self-seeding process, the products may face some problems such as non-uniformity. Many studies have already dealt with finding the most effective parameters in polyol synthesis of silver nanowires. Nishino and Kanno [8] concluded that high PVP concentrations inhibit the growth of silver particles. Moreover, Wang et al. [9] revealed that long-chain PVPs improve the aspect ratio of nanowires. In another study, Amirjani et al. [10] enhanced the aspect ratio of silver nanowires using response surface methodology.
The addition of trace amounts of salts to the polyol synthesis of metal nanostructures has also been shown to influence the morphology of the resulting material. Korte et al. [11] introduced a salt-mediated polyol method to overcome the non-uniformity of the silver nanowires. Xia et al. [12] investigated the addition of NaCl and NaBr salts into the polyol synthesis of silver nanocrystals and concluded that silver nanobar and nanorice are obtainable using these salts. Moreover, Korte et al. [11] concluded that Fe(II) and Fe(III)-containing salts facilitate the growth of one-dimensional Ag nanostructures.
The different values reported for the above-mentioned factors can be explained by the various materials synthesized in these works, as well as the level of other pertinent factors employed in each study. Furthermore, the appropriate level of a factor might also be influenced by the level of the other factors in the synthesis process. In experimental parlance, there might be an interaction between factors in such a process. A survey of the published literature on Ag nanowire synthesis provides limited clues as to whether such interaction between the addition rate of AgNO3 and CuCl2 concentration exists or not. The aim of present work was to identify the interactive role of these two factors in the growth mechanisms of silver nanowires.
Materials and methods
Reagents and solutions
Silver nitrate (AgNO3) was purchased from Scharlau (Spain). Copper chloride dehydrate (CuCl2·2H2O) and ethylene glycol were purchased from Merck. PVP (K-30, MW≈40,000) was purchased from Sigma–Aldrich (USA) and all the reagents were used without further purification.
Synthesizing procedure
In a typical synthesis, 10 mL of ethylene glycol was heated at 160 °C for 1 h with stirring (260 rpm). Then, 80 μL of CuCl2·2H2O/EG solution was added in various concentrations, and the solution was allowed to heat for 15 min. 3 mL of 114 mM PVP/EG was then added to the solution followed by 3 mL of 94 mM AgNO3/EG at different rates. The reaction was stopped after an hour by cooling the reaction chamber to room temperature. Four different tests with distinct values of parameters, listed in Table 1, were done to find out the effect of each factor on the aspect ratio of silver nanowires.
Results and discussion
Characterization
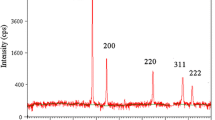
XRD pattern of silver nanowires was obtained and depicted in Fig. 1. The XRD pattern indicates four diffraction peaks observed at 2θ = 38.3°, 44.4°, 64.5° and 77.4°, corresponding to the (111), (200), (220) and (311) planes of the face-centred cubic (fcc) Ag nanowires (JCPDS File No. 04-0783). The UV–Visible absorption spectrum of nanowires was obtained from a dispersed sample in deionized water and was shown in Fig. 2. Two known plasmon resonances, the transverse and longitudinal plasmon bands, are obvious in Fig. 2 at 350 and 390 nm, respectively; which are expected for anisotropic metallic one-dimensional nanostructures [13]. SEM images of silver nanostructures were shown in Fig. 3. AFM image of a single Ag nanowire was shown in Fig. 4.
Stabilizing mechanism of PVP
Multiply-twinned decahedra are the naturally abundant seed morphology, but because of their twin defects, they are also the most reactive. Silver atoms preferentially add to the twin defects of decahedra, leading to uniaxial elongation (Fig. 5). As decahedra grow into pentagonal nanorods, PVP will interact more strongly with the {100} side facets than the {111} facets at the ends of the nanowire. The side surfaces of nanorods are thus passivated by PVP, while the ends remain reactive toward silver atoms. As a result, nanorods rapidly grow into nanowires tens of micrometers long.
The effect of physicochemical parameters
Introducing CuCl2 in the synthesis of Ag nanowires brings forth two distinct phenomena. At first, Cu2+ ions in the solution reduced to Cu1+ in the presence of ethylene glycol as reducing agent. Molecular oxygen present during initial seed formation can adsorb and dissociate on the Ag seeds, blocking sites for further Ag deposition. Cu1+ rapidly scavenges this adsorbed atomic oxygen (Oa), by the Cu1+ being oxidized to Cu2+. Ethylene glycol reduces the Cu2+ back to Cu1+, providing a constant means for oxygen removal which is schemed in Fig. 6.
The second benefit results from Cl− ions in the solution. These ions provide electrostatic stabilization for the initially formed Ag seeds. Introducing Cl− ions helps to reduce the concentration of free Ag+ ions in the solution (and subsequently Ag seeds) through the formation of AgCl nanocrystallites [note that Ksp (AgCl) ≪ Ksp (AgNO3)]. This decrease in free Ag+ during initial Ag seed formation and subsequent slow release of Ag+ effectively mimics the use of a syringe pump to controllably deliver the reagents; it also facilitates the high-yield formation of the thermodynamically more stable multiply-twinned Ag seeds required for wire growth.
A closer study of the results in Fig. 3 reveals that both high concentration of CuCl2 and slow AgNO3 addition rate are important for high-yield synthesis of silver nanowires. When both factors are in their high or low level of values, the results are similar as shown in Table 1. However, when CuCl2 concentration is considerably low, accompanied by rapid injection of AgNO3 (0.4 mmol/min), low aspect ratio silver nanostructures are produced. It was concluded that when the CuCl2 concentration is sufficiently high, the AgNO3 addition rate must be kept low to obtain high aspect ratio silver nanowires.
It is worth to note that when CuCl2 concentration is not sufficiently high (<4 mM), the oxidative etching of small Ag seeds does not take place favourably in the solution. Thus, the formed Ag seeds have various nanostructured shapes that lead to achieving Ag nanowires with low aspect ratio. These results are in compliment with former studies [14, 15]. However, at CuCl2 concentration ≈4 mM, the selective oxidative etching of Ag seeds occurs and consequently, the remaining multiply-twinned particles in the presence of PVP, as capping agent, can effectively grow into one dimensional silver nanostructures (nanowire/nanorod).
Conclusion
Silver nanowires with fairly high aspect ratio were prepared using modified polyol method. The effects of two physicochemical parameters were studied on the aspect ratio of silver nanowires were studied. It was concluded that high concentration of CuCl2 and slow addition rate of AgNO3 are vital for producing high aspect ratio of silver nanowires. It was also found that at low CuCl2 concentrations (<4 mM), the oxidative etching does not take place in a favourable manner, resulting in low aspect ratio nanostructures. These results are applicable for further studies in polyol synthesis of silver nanowires.
References
Lee, J., Sun, F., Lee, J.: Fabrication of large area flexible and highly transparent film by a simple Ag nanowire alignment. J. Exp. Nanosci. 8, 130–137 (2013)
Asgar, M.A., Hasan, M., Huq, M.F., Mahmood, Z.H.: Broadband optical absorption measurement of silicon nanowires for photovoltaic solar cell applications. Int. Nano Lett. 4, 3 (2014)
Amini, N., Gholivand, M.B., Shamsipur, M.: Electrocatalytic determination of traces of insulin using a novel silica nanoparticles—Nafion modified glassy carbon electrode. J. Electroanal. Chem. 714, 70–75 (2014)
Amini, N., Gholivand, M.B., Shamsipur, M.: Nonenzymatic l-lysine amino acid detection using titanium oxide nanoparticles/multi wall carbon nanotube composite electrodes. Electrochim. Acta 123, 569–575 (2014)
Alemi, A., Khademinia, S., Joo, S.W., Dolatyari, M., Bakhtiari, A., Moradi, H., Saeidi, S., Esmaeilzadeh, A.: Hydrothermal synthesis and investigation of optical properties of Nb5+-doped lithium silicate nanostructures. Int. Nano Lett. 4, 4 (2014)
El-Sayed, M.A.: Some interesting properties of metals confined in time and nanometer space of different shapes. Acc. Chem. Res. 34, 257–264 (2001)
Sun, Y., Mayers, B., Herricks, T., Xia, Y.: Polyol synthesis of uniform silver nanowires: a plausible growth mechanism and the supporting evidence. Nano Lett. 3, 955–960 (2003)
Nishino, J., Kanno, Y.: An influence of concentration of polyvinylpyrrolidone on the morphology of silver metal formed from AgNO3 aqueous solution. J. Nanomater. 2008, 1–5 (2008)
Zhu, J.J., Kan, C.X., Wan, J.G., Han, M., Wang, G.H.: High-yield synthesis of uniform Ag nanowires with high aspect ratios by introducing the long-chain PVP in an improved polyol process. J. Nanomater. 2011, 1–7 (2011)
Amirjani, A., Marashi, P., Haghshenas, D.F.: Effect of AgNO3 addition rate on aspect ratio of CuCl2–mediated synthesized silver nanowires using response surface methodology. Colloids. Surf. A Physicochem. Eng. Asp 444, 33–39 (2014)
Korte, K.E., Skrabalak, S.E., Xia, Y.: Rapid synthesis of silver nanowires through a CuCl- or CuCl2-mediated polyol process. J. Mater. Chem. 18, 437–441 (2008)
Wiley, B., Sun, Y., Xia, Y.: Synthesis of silver nanostructures with controlled shapes and properties. Acc. Chem. Res. 40, 1067–1076 (2007)
Murphy, C.J., Jana, N.R.: Controlling the aspect ratio of inorganic nanorods and nanowires. Adv. Mater. 14, 80–82 (2002)
Xia, Y., Xiong, Y., Lim, B., Skarbalak, S.E.: Shape-controlled synthesis of metal nanocrystals: simple chemistry meets complex physics? Angew. Chem. Int. Ed. 48, 60–103 (2009)
Tang, X., Tsuji, M., Jiang, P., Nishio, M., Jang, S.M., Yoon, S.H.: Rapid and high-yield synthesis of silver nanowire using air-assisted polyol method chloride ions. Coll. Surf. A Physicochem. Eng. Asp 338, 33–39 (2009)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under license to BioMed Central Ltd.Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Amirjani, A., Marashi, P. & Fatmehsari, D.H. The effects of physicochemical parameters on the synthesis of silver nanowires via polyol method. Int Nano Lett 4, 108 (2014). https://doi.org/10.1007/s40089-014-0108-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40089-014-0108-5