Abstract
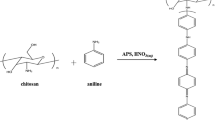
Polyaniline as a conductive polymer and chitosan as a natural polymer have been reacted with formaldehyde as grafting agent and potassium persulfate as an initiator. The effect of using specific primer, different ratio of monomers and the solubility of synthesized copolymer has been studied and analyzed. Characterization of this new copolymer were occurred by Fourier transform infrared spectroscopy, UV–visible, scanning electron microscopy and differential scanning calorimetry technique.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Conducting polymers such as polyaniline (PAni) have drawn considerable interest for their wide applications. Polyaniline is one of the most attractive conducting polymers due to the high conductivity and good stability. PAni as a conductive polymer has attracted a lot of attention, due to simple preparation and doping procedure, good environmental stability, relatively high conductivity and low cost and also wide spectrum of applications.
The nanofibrillar morphology significantly improves the performance of polyaniline in many conventional applications involving polymer interactions with its environment. The high conjugated polymeric structure of polyaniline produces new nanoscale phenomena that are not accessible with current inorganic systems. Most studies in the field were devoted to the preparation of PANi/noble metal composites. Conducting polymers find applications in fields like: sensors, electro catalysts, microelectronics, electromagnetic shielding, rechargeable batteries and controlling systems. [1–4].
Because of these problems, many methods are reported. Grafting with other polymers having good mechanical and physical properties is one of them. Many potential applications of PAni are demonstrated recently [2, 5–7]. The polymers should be insoluble in whole range of pH to gain some of the applications. One way to overcome these problems is chemical grafting of PAni onto the radiation cross-linked chitosan (CS). Polyaniline is of great interest because of good conductibility and ease of formation and is a good candidate for this research. Chitosan is a polysaccharide derived from the chitin of crustaceans. The properties of cationic polysaccharides are the low toxicity and good biocompatibility that make them interesting for study as a drug excipient [8]. The nitrogen electrons in the amino and N-acetyl amino groups can establish dative bonds with transition metal ions and some hydroxyl groups in CS may function as donors [9]. Chitosan can chelate metal ions by itself, especially those listed as transition metals. It can apply as a matrix for immobilization of enzymes too [10]. Due to its physical and chemical properties, CS is used in different products such as pharmaceutical and cosmetic products for water treatment and plant protection. In different applications, different properties of CS are required [11]. Therefore, the aim of this study is to improve physico-chemical properties of PAni by varying the grafting ratio of CS. Because of these reasons, the results were investigated using FT-IR, UV–Vis, SEM, and DSC techniques.
Methods
The CS, acetic acid, hydrochloric acid (37 % purification), N-methyl pyrrolidine (NMP) and ammonium persulfate (APS) with 98 % purification prepared from Merck Company and used without further purification.
All infrared spectra were obtained from samples in KBr pellets using a Bruker Tensor27 Ger FT-IR spectrophotometer. 1H NMR spectrum was taken by a Bruker WP 200 SY spectrometer operating at 250 MHz in DMSO. To evaluate the samples, UV–vis has been used (model Perkin Elmer Lamer Lambda25) too. Scanning electron microscopy (SEM) instrument models were XL30, Philips, Holland. Also the thermal behavior of the polymers was obtained from the STA STA S-1500 Scinco model made in Korea. Finally, the samples were mixed together with Alfa magnetite stirrer (model: Hs-860).
Preparation of polyaniline
One millilitre of freshly distilled aniline was dissolved in 30 ml of hydrochloric acid (1 M) and was brought to a temperature of 4–0 °C in an ice bath. Then, a certain amount of KPS was added to 20 ml of hydrochloric acid (1 M) and stirred. The prepared solution was added to the first solution drop by drop. A few minutes after injection of the oxidant, the solution turned bluish-green color. The color intensity of the reaction was greater by the time. After 5 h of stirring, the solution was filtered through filter paper. Greenish-black deposits remaining on the filter paper were washed by water and were dried in an electric oven at 60 °C for 12 h finally. Quantitative details of this process are given in Table 1.
Preparation of copolymer
A certain amount of CS was dissolved in 20 ml acetic acid solution (2 % w/w) and stirred for 1 h. Then 10 ml of KPS solution (prepared in last section) was added to the first solution drop by drop at 5 °C. Again, a solution of certain amount of freshly distilled aniline in 10 ml of distilled water was prepared and was added to the final solution drop by drop over 20 min. Stirring was continued for 12 h. Then, the solution was filtered and dried. Dark green to black powder is the expected copolymer.
Grafting of copolymer
A certain amount of chitaline (as prepared in last section) was dissolved in 20 ml of 2 M ammonia and stirred at room temperature for 1 h. Then, it was filtered and the precipitate was dried in an oven at 60 °C for 3 h. The, the obtained solid was stirred with 2 ml of 1 M acetic acid until fully dissolved. So by adding excess amounts of acetic acid the pH raised to four. Meanwhile by adding drop by drop a certain amount of formaldehyde, the grafting process is started. Stirring was continued for 12 h at room temperature. The solid was filtered, washed with distilled water and dried at 60 °C for 12 h. A dark green powder was obtained including the chitosan grafted polyaniline (chit-g-c-PAni). Different ratios of aniline and formaldehyde were examined.
Result and discussion
1H NMR analysis of CS-graft-PAni copolymer
The 1H NMR spectrum confirmed the grafting of PAni chain onto CS. In Fig. 1, 1H NMR spectrum of the final CS-graft-PAni copolymers, marked peak in the ppm 1.71 ppm, belongs to the CH2 protons along the chain. Wide peak in the 2.5 ppm indicates the CH protons of CS that overlap with protons in the DMSO. Wide peak in 3.7 ppm is the especial peak of hydroxyl groups along the chain that are overlapping with H2O. There is also a broad peak in the region between 6.97 and 7.69 ppm belonging to the protons of aromatic ring and benzene groups in PAni. All these evidence affirmed the structure of CS-graft-PAni copolymers.
Fourier transforms infrared analysis of PAni and CS-graft-PAni copolymer
The FTIR spectrum of PAni is shown in Fig. 2. The peak at 3,452 cm−1 is related to N–H stretching vibrations, area of 1,560–1,650 cm−1 is related to NH flexural vibrations and the peak at 1,560 cm−1 is attributed to quinoid (Q) rings vibrations of PAni. The peak at 1,481 cm−1 is due to benzoid vibrations. Also the vibrations of N=Q=N have characteristic absorption at 1,132 cm−1. Aromatic C–N stretching vibrations appear at 1,298 cm−1 and the peak at 504–879 cm−1 is due to CH bending vibrations of substituted benzene ring.
Various vibrations of final grafted product (Fig. 3) are also analyzed. The wide peak observed in the area of 3,200–3,500 cm−1 is due to the overlapping between O–H stretching vibrations in CS and NH stretching vibrations in PAni. Also there are peaks at the areas of 2,858 and 2,925 cm−1 which are owned to CH stretching vibrations and the peak at 1,636 cm−1 is due to C=O stretching vibrations of CS. The area at 11,459 cm−1 belongs to the C=C and C=N stretching vibrations in benzoid rings of PAni. Stretching vibrations of the aromatics are approximately at 1,301 cm−1.
As can be seen, stretching vibrations of N=Q=N, after linking CS with PAni, shift from 1,132 to 1,040 cm−1. NH vibration of CS was seen in the region around the peak at 1,655 cm−1 too.
TGA-DSC and SEM characterization of CS-graft-PAni copolymer
Figure 4 is the STA spectrum of graft copolymer. As can be seen the polymer begins to soften from the temperature of about 50 °C, and it continues until the temperature of 210 °C. This issue is caused by the loosening of connections between PAni and CS (Fig. 5). At this point, the player loses almost 10 % of its weight. This mass loss can be due to the loss of moisture and solvent of polymer. Degradation begins at 210 °C and continues to 500 °C. The reason is PAni and CS chain degradation, caused 50 % mass reduction. Complete degradation occurs above 500 °C temperature and continues to 600 °C. Instead of burning, the polymer remains <10 % ash.
Conclusion
The information of 1H NMR, FT-IR, STA and SEM spectra was produced the structure of synthesized copolymer. Solubility test results showed that the synthesized copolymer link is sparingly soluble in solvents tested, and also electrical conductivity of grafted copolymer is less than Ani with unclear reason. Besides the chemical structure, many factors including the morphology and shape of the polymer chain may affect the electrical conductivity, and also molar ratio of Ani and APS is effective on the electrical conductivity of the synthesized copolymer as shown in Table 1. The electrical conductivity increases when the initiator amount is greater than the Ani. In general, high value of initiator or high temperature has a positive effect on the polymerization rate, but it reduces the degree of polymerization. Also half of the CS amount led to a slight increase in electrical conductivity but the quality of the synthesized copolymer would not be good. It is known that the electrical properties of the conducting polymer depend on the size and shape of the particles.
References
Sedaghat, S.: J. Nanostructure Chem. 1(2), 90–94 (2010)
Chen, C.H.: J. Appl. Polym. Sci. 89, 2142–2148 (2003)
Yin, W., Li, J., Li, Y., Wu, Y., Gu, T.: Polym. Int. 42, 276–280 (1997)
Lee, Y.M., Kim, J.H., Kang, J.S., Ha, S.Y.: Macromolecules 33, 7341 (2000)
Venkatachalam, S., Prabhakaran, P.V.: Synth. Met. 97, 141 (1998)
Lee, Y.M., Nam, S.Y., Ha, S.Y.: J. Membr. Sci. 159, 41–46 (1999)
Agbor, N.E., Petty, M.C., Monkman, A.P.: Sens. Actuators B 28, 173 (1995)
Yang, S., Tirmizi, S.A., Burns, A., Barney, A.A., Risen Jr, W.M.: Synth. Met. 32, 191–200 (1989)
Ismail, Y.A., Shin, S.R., Shin, K.M., Yoon, S.G., Shon, K., Kim, S.I., Kim, S.J.: Sens. Actuators B Chem. 129, 834–840 (2008)
Shi, Q.-H., Tian, Y., Bai, S., Sun, Y.: Biochem Eng J 16, 317 (2003)
Sedaghat, S.: J. Nanostructure Chem. 2(1), 53–57 (2011)
Acknowledgments
We warmly thank Islamic Azad University for any supports.
Conflict of interest
As we can see in the results the synthesized copolymer has improved properties and can be develpoed in future for some applications.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under license to BioMed Central Ltd.Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Sedaghat, S. Synthesis and characterization of new biocompatible copolymer: chitosan-graft-polyaniline. Int Nano Lett 4, 99 (2014). https://doi.org/10.1007/s40089-014-0099-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40089-014-0099-2