Abstract
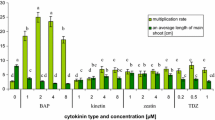
Cytokinins (CKs) are regulatory molecules that affect a number of important structural and functional aspects in plants. In this study, the effect of four CKs including the Thidiazuron (TDZ), Kinetin (Kin), 6-Benzylaminopurine (BA) and 2-isopentenyladenine (2ip) (1, 2, 4, and 8 μM) on the shoot proliferation, photosynthetic pigments (chlorophyll a and b and carotenoids), flavonoids, anthocyanins and phenolics, antioxidant activity and survival rate of Venus flytrap plantlets was investigated. The results showed that the increasing the concentration of the CKs resulted in increase in plantlets root and leaf growth as well as photosynthetic pigments content. The leaf and root growth and also photosynthetic pigments content (chlorophyll a and b and carotenoids) were most effective in TDZ-supplemented medium. The shoots from medium supplemented with 2 µM TDZ demonstrated the highest production of total flavonoids, anthocyanins and phenolics as well as antioxidant activity, which was 1.95, 0.23, 8.51 mg/g FW and 28.62 µg/ml, respectively. The highest shoot proliferation (7.5/explant) was observed at the 8 μM TDZ, which showed a 40% increase in compared to cultures with its least level. Regression analysis justified 81.02% and 70.2% of changes in survival rate in both TDZ and Kin by root length and total chlorophyll content. Correlation analyses revealed that regenerated plantlets with more growth indices in organs, metabolites and antioxidant activity had better survival rates. It seems that more developed organs, photosynthetic pigments and secondary metabolites content as well as antioxidant activity in micropropagated plantlets contributes to better ex vitro survival.
Similar content being viewed by others
References
Hedrich R, Neher E (2018) Venus flytrap: how an excitable, carnivorous plant works. Trends Plant Sci 23:220–234
Gaascht F, Dicato M, Diederich M (2013) Venus flytrap (Dionaea muscipula Solander ex Ellis) contains powerful compounds that prevent and cure cancer. Front Oncol 3:202–210
Chokheli VA, Dmitriev PA, Rajput VD, Bakulin SD, Azarov AS, Varduni TV, Stepanenko VV, Tarigholizadeh S, Singh RK, Verma KK, Minkina TM (2020) Recent development in micropropagation techniques for rare plant species. Plants 9:1733
Aremu AO, Bairu MW, Szüčová L, Finnie JF, van Staden J (2012) The role of meta-topolins on the photosynthetic pigment profiles and foliar structures of micropropagated ‘Williams’ bananas. J plant physiol 169:1530–1541
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Hutchinson JF (1984) In vitro propagation of Dionaea muscipula Ellis (Venus flytrap). Sci Hortic 22:189–194
Jang GW, Kim KS, Park RD (2003) Micropropagation of Venus flytrap by shoot culture. Plant Cell Tissue Organ Cult 72:95–98
Lichtenthaler HK (1987) Chlorophylls, carotenoids: pigments of photosynthetic biomembranes. Methods enzymol 148:350–382
Shen Y, Jin L, Xiao P, Lu Y, Bao J (2009) Total phenolics, flavonoids, antioxidant capacity in rice grain and their relations to grain color, size and weight. J Cereal Sci 49:106–111
Jeong JH, Jung H, Lee SR, Lee HJ, Hwang KT, Kim TY (2010) Anti-oxidant, anti-proliferative and anti-inflammatory activities of the extracts from black raspberry fruits and wine. Food Chem 123:338–344
Efroni I, Han SK, Kim HJ, Wu MF, Steiner E, Birnbaum KD, Hong JC, Eshed Y, Wagner D (2013) Regulation of leaf maturation by chromatin-mediated modulation of cytokinin responses. Dev Cell 24:438–445
Piątczak E, Grzegorczyk-Karolak I, Wysokińska H (2014) Micropropagation of Rehmannia glutinosa Libosch.: production of phenolics and flavonoids and evaluation of antioxidant activity. Acta Physiol Plant 36:1693–1702
Grevenstuk T, Coelho N, Gonçalves S, Romano A (2010) In vitro propagation of Drosera intermedia in a single step. Biol Plant 54:391–394
Agarwala B, Azam FMS, Khatun MA, Rahman F, Rahmatullah M (2010) Simultaneous shoot regeneration and rhizogenesis of Wedelia chinensis for in vitro clonal propagation. Am-Eurasian J Sustain Agric 4:65–69
Weremczuk-Jeżyna I, Kuźma L, Kiss AK, Grzegorczyk-Karolak I et al (2018) Effect of cytokinins on shoots proliferation and rosmarinic and salvianolic acid B production in shoot culture of Dracocephalum forrestii WW Smith. Acta Physiol Plant 40:1–10
Gan S, Amasino RM (1996) Cytokinins in plant senescence: From spray and pray to clone and play. BioEssays 18:557–565
Park H, Kim DH, Saini RK, Gopal J, Keum YS, Sivanesan I et al (2019) Micropropagation and quantification of bioactive compounds in Mertensia maritima (L.) Gray. Int J Mol Sci 20:2141
Talla SK, Panigrahy M, Kappara S, Nirosha P, Neelamraju S, Ramanan R (2016) Cytokinin delays dark-induced senescence in rice by maintaining the chlorophyll cycle and photosynthetic complexes. J Exp Bot 67:1839–1851
Youssef NM, Taha LS, El-Khalek A, Nagy S (2021) Secondary metabolites characterization of in vitro propagated Antigonon leptopus cultures. Egypt J Chem 64(2):923–932
Carimi F, Terzi M, De Michele R, Zottini M, Schiavo FL (2004) High levels of the cytokinin BAP induce PCD by accelerating senescence. Plant Sci 166:963–969
Amoo SO, Van Staden J (2013) Influence of plant growth regulators on shoot proliferation and secondary metabolite production in micropropagated Huernia hystrix. Plant Cell Tiss Organ Cult 112:249–256
Lee Y, Lee DE, Lee HS, Kim SK, Lee WS, Kim SH, Kim MW (2011) Influence of auxins, cytokinins, and nitrogen on production of rutin from callus and adventitious roots of the white mulberry tree (Morus alba L.). Plant Cell Tiss Organ Cult 105:9–19
Ali M, Abbasi BH (2014) Thidiazuron-induced changes in biomass parameters, total phenolic content, and antioxidant activity in callus cultures of Artemisia absinthium L. Appl Biochem Biotechnol 172:2363–2376
Grzegorczyk-Karolak I, Kuźma Ł, Wysokińska H (2015) The effect of cytokinins on shoot proliferation, secondary metabolite production and antioxidant potential in shoot cultures of Scutellaria alpina. Plant Cell Tiss Organ Cult 122:699–708
Kaur P, Singh K (2015) Influence of growth regulators on physiology and senescence of cut stemsof Chrysanthemum (Chrysanthemum morifolium Ramat) Var. thai ching queen. Int J Allied Pract Res Rev 2:31–41
Oliveira LM, Paiva R, Santana JR, Pereira FD, Nogueira RC, Silva LC (2010) Effects of cytokinins on in vitro mineral accumulation and bud development in Annona glabra L. Cienc Agrotecnologia 34:1439–1445
Trueman SJ (2018) Cytokinin and auxin effects on survival and rooting of Eucalyptus pellita and E. grandis × E. pellita cuttings. Rhizosphere 6:74–76
Oliveira LM, Paiva R, de Santana JR, Alves E, Nogueira RC, Pereira FD (2008) Effect of cytokinins on in vitro development of autotrophism and acclimatization of Annona glabra L. In Vitro Cell Dev Biol Plant 44:128–135
Sudha CG, Krishnan PN, Pushpangadan P, Seeni S (2005) In vitro propagation of Decalepis arayalpathra, a critically endangered ethnomedicinal plant. In Vitro Cell Dev Biol Plant 41:648–654
Kanechi M, Ochi M, Abe M, Inagaki N, Maekawa S (1998) The effects of carbon dioxide enrichment, natural ventilation, and light intensity on growth, photosynthesis, and transpiration of cauliflower plantlets cultured in vitro photoautotrophically and photomixotrophically. J Am Soc Hortic Sci 123:176–181
Acknowledgements
We appreciate the financial support from the Shahed University, Tehran, Iran.
Author information
Authors and Affiliations
Contributions
A.R contributed to conceptualization, supervision, methodology, and writing. MN contributed to methodology, investigation, and formal analysis. IR contributed to advision.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Noormohammadi, M., Rezaei, A. & Rohollahi, I. Effect of cytokinins on shoot proliferation, metabolite production and antioxidant activity in Venus flytrap (Dionaea muscipula Ellis). Proc. Natl. Acad. Sci., India, Sect. B Biol. Sci. (2024). https://doi.org/10.1007/s40011-024-01555-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40011-024-01555-x