Abstract
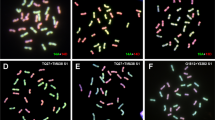
In newly formed allopolyploids, meiotic instability in early generations contributes to reduced fertility, but less is known about their mitotic accuracy and somatic instability. Here, the authors report somatic numerical chromosome variation (somatic aneuploidy) among different individuals of newly synthesized wheat–Aegilops triuncialis amphiploids (AABBDDUtUtCtCt genome) over three successive generations. In total, 21 seeds in the F2 to F4 generations descended from three different F1 hybrids (2n = 5x = 35, ABDUtCt genome) were analyzed by chromosome counting and fluorescent in situ hybridization. Somatic aneuploidy was detected in 17 (81%) investigated individuals. Somatic chromosome numbers of the cells (among and within the evaluated individuals) ranged from 62 to 70. Furthermore, flow cytometry revealed variation in genome size within and between individual amphiploids that may be due to variable chromosome numbers. There was no evidence that somatic aneuploidy leads to stable cytotypes, at least in the early generations. The authors conclude that both newly formed status and large genome sizes may contribute to somatic and meiotic instability in synthetic wheat–Aegilops triuncialis amphiploids.



Similar content being viewed by others
References
Schubert I, Vu GT (2016) Genome stability and evolution: attempting a holistic view. Trends Plant Sci 21:749–757
Holland AJ, Cleveland DW (2012) Losing balance: the origin and impact of aneuploidy in cancer: ‘exploring aneuploidy: the significance of chromosomal imbalance’ review series. EMBO Rep 13:501–514
De Storme N, Mason A (2014) Plant speciation through chromosome instability and ploidy change: cellular mechanisms, molecular factors and evolutionary relevance. Curr Plant Biol 1:10–33
Gou X, Bian Y, Zhang A, Zhang H, Wang B, Lv R, Li J, Zhu B, Gong L, Liu B (2018) Transgenerationally precipitated meiotic chromosome instability fuels rapid karyotypic evolution and phenotypic diversity in an artificially constructed allotetraploid wheat (AADD). Mol Biol Evol 35:1078–1091
Kostoff D (1938) Studies on polyploid plants. Irregularities in the mitosis and polyploidy induced by colchicine and acenaphthene. Curr Sci 6:549–552
Lim KY, Soltis DE, Soltis PS, Tate J, Matyasek R, Srubarova H, Kovarik A, Pires JC, Xiong Z, Leitch AR (2008) Rapid chromosome evolution in recently formed polyploids in Tragopogon (Asteraceae). PLoS ONE 3:e3353
Wright KM, Pires JC, Madlung A (2009) Mitotic instability in resynthesized and natural polyploids of the genus Arabidopsis (Brassicaceae). Am J Bot 96:1656–1664
Wolters AMA, Schoenmakers HCH, Kamstra S, Jv E, Koornneef M, Jong JHd (1994) Mitotic and meiotic irregularities in somatic hybrids of Lycopersiconesculentum and Solanumtuberosum. Genome 37:726–735
Sharma CBSR (1990) Chemically induced aneuploidy in higher plants. Mutagenesis 5:105–125
Phillips RL, Kaeppler SM, Olhoft P (1994) Genetic instability of plant tissue cultures: breakdown of normal controls. Proc Natl Acad Sci 91:5222–5226
Damato F (1985) Cytogenetics of plant cell and tissue cultures and their regenerates. Crit Rev Plant Sci 3:73–112
McClintock B (1993) The significance of responses of the genome to challenge. World Scientific, Singapore
Wendel JF, Lisch D, Hu G, Mason AS (2018) The long and short of doubling down: polyploidy, epigenetics, and the temporal dynamics of genome fractionation. Curr Opin Genet Dev 49:1–7
Mirzaghaderi G, Mason AS (2017) Revisiting pivotal-differential genome evolution in wheat. Trends Plant Sci 22:674–684
Freeling M, Scanlon MJ, Fowler JF (2015) Fractionation and subfunctionalization following genome duplications: mechanisms that drive gene content and their consequences. Curr Opin Genet Dev 35:110–118
Pavlíková Z, Paštová L, Münzbergová Z (2017) Synthetic polyploids in Viciacracca: methodology, effects on plant performance and aneuploidy. Plant Syst Evol 303:827–839
Fakhri Z, Mirzaghaderi G, Ahmadian S, Mason AS (2016) Unreduced gamete formation in wheat × Aegilops spp. hybrids is genotype specific and prevented by shared homologous subgenomes. Plant Cell Rep 35:1143–1154
Mirzaghaderi G, Abdolmalaki Z, Zohouri M, Moradi Z, Mason AS (2017) Dynamic nucleolar activity in wheat × Aegilops hybrids: evidence of C-genome dominance. Plant Cell Rep 36:1277–1285
Abdolmalaki Z, Mirzaghaderi G, Mason AS, Badaeva ED (2019) Molecular cytogenetic analysis reveals evolutionary relationships between polyploid Aegilops species. Plant Syst Evol 305:459–475
Mirzaghaderi G, Houben A, Badaeva ED (2014) Molecular-cytogenetic analysis of Aegilopstriuncialis and identification of its chromosomes in the background of wheat. Mol Cytogenet 7:91
Loureiro J, Rodriguez E, Doležel J, Santos C (2007) Two new nuclear isolation buffers for plant DNA flow cytometry: a test with 37 species. Ann Bot 100:875–888
Doležel J, Bartoš J (2005) Plant DNA flow cytometry and estimation of nuclear genome size. Ann Bot 95:99–110
Bennett M, Leitch I (2011) Nuclear DNA amounts in angiosperms: targets, trends and tomorrow. Ann Bot 107:467–590
Chester M, Gallagher JP, Symonds VV, da Silva AVC, Mavrodiev EV, Leitch AR, Soltis PS, Soltis DE (2012) Extensive chromosomal variation in a recently formed natural allopolyploid species, Tragopogonmiscellus (Asteraceae). Proc Natl Acad Sci 109:1176–1181
Xiong Z, Gaeta RT, Pires JC (2011) Homoeologous shuffling and chromosome compensation maintain genome balance in resynthesized allopolyploid Brassicanapus. Proc Natil Acad Sci 108:7908–7913
Mason AS, Nelson MN, Takahira J, Cowling WA, Alves GM, Chaudhuri A, Chen N, Ragu ME, Dalton-Morgan J, Coriton O (2014) The fate of chromosomes and alleles in an allohexaploid Brassica population. Genetics 197:273–283
Soltis DE, Visger CJ, Marchant DB, Soltis PS (2016) Polyploidy: pitfalls and paths to a paradigm. Am J Bot 103:1146–1166
Zhang H, Bian Y, Gou X, Dong Y, Rustgi S, Zhang B, Xu C, Li N, Qi B, Han F (2013) Intrinsic karyotype stability and gene copy number variations may have laid the foundation for tetraploid wheat formation. Proc Natl Acad Sci 110:19466–19471
King RW (2008) When 2 + 2 = 5: the origins and fates of aneuploid and tetraploid cells. Biochim Biophys Acta 1786:4–14
Jefford CE, Irminger-Finger I (2006) Mechanisms of chromosome instability in cancers. Crit Rev Oncol/Hematol 59:1–14
de Sousa JT, Allen SK, Wolfe BM, Small JM (2017) Mitotic instability in triploid and tetraploid one-year-old eastern oyster, Crassostreavirginica, assessed by cytogenetic and flow cytometry techniques. Genome 61:79–89
Kaur D, Singhal VK (2019) Meiotic abnormalities affect genetic constitution and pollen viability in dicots from Indian cold deserts. BMC Plant Biol 19:10
Mirzaghaderi G, Fathi N (2015) Unreduced gamete formation in wheat—Aegilopstriuncialis interspecific hybrids leads to spontaneous complete and partial amphiploids. Euphytica 206:67–75
Wang D, Ling L, Zhang W, Bai Y, Shu Y, Guo C (2018) Uncovering key small RNAs associated with gametocidal action in wheat. J Exp Bot 69:4739–4756
Endo TR, Tsunewaki K (1975) Sterility of common wheat with Aegilops triuncialis cytoplasm. J Hered 66:13–18
Murata K, Watanabe S, Tsujimoto H, Nasuda S (2018) Cytological observation of chromosome breakage in wheat male gametophytes caused by gametocidal action of Aegilopstriuncialis-derived chromosome 3Ct. Genes Genet Syst 93:111–118
Tsujimoto H, Tsunewaki K (1985) Gametocidal genes in wheat and its relatives. II. Suppressor of the chromosome 3C gametocidal gene of Aegilopstriuncialis. Can J Genet Cytol 27:178–185
Mirzaghaderi G, Karimzadeh G, Hassani HS, Jalali-Javaran M, Baghizadeh A (2010) Cytogenetic analysis of hybrids derived from wheat and Tritipyrum using conventional staining and genomic in situ hybridization. Biol Plant 54:252–258
Acknowledgements
This work was supported by the University of Kurdistan and Iran National Science Foundation (INSF) (Grant 95826690).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Significance statement Somatic and meiotic instabilities were observed in newly synthetized wheat–Aegilops triuncialis amphiploids (AABBDDUtUtCtCt genome) over three successive generations, which may be caused by both nascent status and large genome sizes.
Rights and permissions
About this article
Cite this article
Amjadian, S., Mirzaghaderi, G. Somatic and Meiotic Instabilities Cause Hypo-aneuploidy in Synthesized Wheat–Aegilops triuncialis Amphiploids. Proc. Natl. Acad. Sci., India, Sect. B Biol. Sci. 90, 997–1004 (2020). https://doi.org/10.1007/s40011-020-01169-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40011-020-01169-z