Abstract
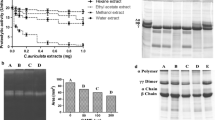
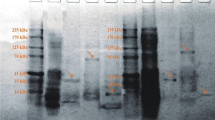
Plant extracts are extensively used in folk medicine to treat the snake bites. In the present study, anti-venom potential of Canthium parviflorum root extracts against Naja naja venom was studied by in vitro and in vivo methods. Ethyl acetate and methanolic root extracts were analysed for in vitro neutralization of 5′-nucleotidase, phospholipase A2, acetylcholinesterase, phosphodiesterase, hyaluronidase, phosphomonoesterase and protease enzyme activities. Neutralization of pharmacological activities like fibrinogenolytic, and direct and indirect haemolytic activities with active methanol extract was performed. Lethal toxicity determination and its inhibition by root extract were carried out in vitro on chick embryo and in vivo on mice model. Further, venom induced edema, myotoxicity and its neutralization were carried out in the mice model. Both extracts inhibited all enzyme activities in a dose dependent manner with various IC50 values. However, only methanol extract effectively neutralized protease activity. Active methanol extract significantly neutralized all the pharmacological activities at various concentrations. Lethal dose (LD50) of venom was 2.5 µg/egg and effective dose of plant was 0.79 mg/egg for 2 LD50 of the venom. LD50 value of venom was 0.38 mg/kg body weight of mice and survival time was prolonged due to plant extract. At 1:10 and 1:20 of venom: plant extract concentration (w/w) significantly neutralized edema and myotoxic effects respectively in mice model. The present study revealed the potential of C. parviflorum root extract for its anti-venom properties and the plants could be considered as a potential source of anti-venom phytoconstituents.








Similar content being viewed by others
References
Bawaskar HS (2004) Snake venoms and antivenom issues: critical supply issues. J Assoc Physicians India 52:11–13
Lee CC, Chang CC (1966) Modes of actions of purified toxins from elapid venoms on neuromuscular transmission. Mem Inst Butantan 33:555–572
Watt MD, Theakston DRG, Hayes CG (1986) Positive response to endrophonium in patients with neurotoxic envenoming by cobras (Naja naja philippinensis). N Engl J Med 315:1444–1448. https://doi.org/10.1056/NEJM198612043152303
Mukherjee AK, Maity CR (2002) Biochemical composition, lethality and pathophysiology of venom from two cobras-Naja naja and Naja kaouthia. Comp Biochem Physiol B: Biochem Mol Biol 131:125–132
Vishwanath BS, Kini RM, Gowda TV (1987) Characterization of three edema inducing phospholipase A2 enzymes from Habu (Trimeresurus flavoviridis) venom and their interaction with the alkaloid aristolochic acid. Toxicon 25:501–515. https://doi.org/10.1016/0041-0101(87)90286-8
Cannon R, Ruha AM, Kashani J (2008) Acute hypersensitivity reactions associated with administration of crotalidae polyvalent immune Fab antivenom. Ann Emerg Med 51:407–411. https://doi.org/10.1016/j.annemergmed.2007.09.036
Gutierrez JM, Avila C, Rojas E, Cerdas L (1988) An alternative in vitro method for testing the potency of the polyvalent antivenom produced in Costa Rica. Toxicon 26:411–413. https://doi.org/10.1016/0041-0101(88)90010-4
Bawaskar HS, Bawaskar PH (2002) Profile of snakebite envenoming in western Maharashtra India. Trans R Soc Trop Med Hyg 96:79–84
Alam MI (2014) Inhibition of toxic effects of Viper and Cobra venom by Indian medicinal plants. Pharmacol Pharm 5:828–837. https://doi.org/10.4236/pp.2014.58093
Hiremath VT, Taranath TC (2010) Traditional phytotherapy for snake bites by tribes of Chitradurga District, Karnataka, India. Ethnobot Leafl 14:120–125
Harborne JB (1973) Methods of plant analysis. In: Phytochemical methods, 2nd edn. Chapman and Hall, London, pp 132
Rowe M, de Gast GC, Platts-Mills Asherson TA, Webster GL, Johnson SM (1980) Lymphocyte 5′-nucleotidase in primary hypogammaglobulinaemia and cord blood. Clin Exp Immunol 39:337–343
Tan NH, Tan CS (1988) Acidimetric assay of phospholipase A2 using egg yolk suspension as substrate. Anal Biochem 170:282–288
Ellman GL, Courtney KD, Andres VJ, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95. https://doi.org/10.1016/0006-2952(61)90145-9
Lo TB, Chen YH, Lee CY (1966) Chemical studies of Formosan cobra (N. naja atra) venom. Part 1. Chromatographic separation of crude venom on CM-Sephadex and preliminary protection by Mikania laevigata (guaco) extract against the toxicity of Philodryas olfersii snake venom’ characterization of its components. J Chin Chem Soc 13:165–177
Pukrittayakamee S Warrell, Desakorn DA, McMichael V, White AJ, Bunnag D (1988) The hyaluronidase activities of some Southeast Asian snake venoms. Toxicon 26:629–637
Bessey OA, Lowry OH, Brock MJ (1946) A method for the rapid determination of alkaline phosphates with five cubic millimeters of serum. J Biol Chem 164:321
Greenberg DM (1955) Plant proteolytic enzymes: methods in enzymology. In: Colowick SP, Kalpan NO (eds) Academic Press Inc., New York, pp 54–64
Ouyang C, Teng CM (1976) Fibrinogenolytic enzymes of Trimeresurus mucrosquamatus venom. Biochim Biophys Acta 420:298–308
Balu S, Alagesaboopathy C (1995) Anti-snake venom activities of some species of andrographis wall. Ancient Sci Life 14:187–190
Sells PG, Ioannou P, Theakston RDG (1998) A humane alternative to the measurement of the lethal effects (LD50) of non-neurotoxic venoms using hens’ eggs. Toxicon 36:985–991
Theakston RDG, Reid HA (1983) Development of simple standard assay procedures for the characterization of snake venoms. Bull World Health Organ 61:949–956
Meier J, Theakston RDG (1986) Approximate LD50 determination of snake venoms using eight to ten experimental animals. Toxicon 24:395–401
Gutierrez JM, Arce V, Brenes F, Chaves F (1990) Changes in myofibrillar components after skeletal muscle necrosis induced by a myotoxin isolated from the venom of the snake Bothrops asper. Exp Mol Pathol 52:25–37
Kumarapppan C, Jaswanth A, Kumarasunderi K (2011) Antihaemolytic and snake venom neutralizing effect of some Indian medicinal plants. Asian Pac J Trop Med 11:743–747. https://doi.org/10.1016/S1995-7645(11)60185-5
Dhananjaya BL, Zameer F, Girish KS, Cletus D’Souza JM (2011) Antivenom potential of aqueous extract of stem bark of Mangifera indica L. against Daboia russellii (Russell’s viper) venom. IJBB 48:175–183
Richard Lobo ISR, Punitha K Rajendran, Shirwaikar Arun, Shirwaikar Annie (2006) Preliminary study on the antisnake venom activity of alcoholic root extract of Clerodendrum viscosum (Vent.) in Naja naja venom. Nat Prod Sci 12:153–156
van der Valk T, van der Meijden A (2014) Toxicity of scorpion venom in chick embryo and meal worm assay depending on the use of the soluble fraction versus the whole venom. Toxicon 88:38–43. https://doi.org/10.1016/j.toxicon.2014.06.007 (Epub 2014 Jun 19)
Mahanta M, Mukherjee AK (2001) Neutralisation of lethality, myotoxicity and toxic enzymes of Naja kaouthia venom by Mimosa pudica root extracts. J Ethnopharmacol 75:55–60
Mors WB, Nascimento MC, Pereira BM, Pereira NA (2000) Plant natural products active against snake-bite the molecular approach. Phytochemistry 55:627–642
Acknowledgements
The authors thank Jain University for their financial support and infrastructure facilities to carry out the research work. They are thankful to Nargund College of Pharmacy, Karnataka, India to provide facilities to carry out the animal studies. They would like to acknowledge the Institute of Animal Health and Veterinary Biological (IVHVB), Bangalore, Karnataka, India to provide the facilities and valuable suggestions to carry out egg experiments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report that they have no conflicts of interest to publish this manuscript.
Additional information
Significance of paper The present study demonstrated the anti-venom potential of C. parvifloum root extracts against the Naja naja venom. The outcome of the present study is encouraging and intended to bridge the gap between traditional knowledge.
Rights and permissions
About this article
Cite this article
Shrikanth, V.M., Janardhan, B. & More, S.S. Anti-Venom Potential of Canthium parviflorum Against Naja naja Venom by In Vitro and In Vivo Studies. Proc. Natl. Acad. Sci., India, Sect. B Biol. Sci. 89, 483–492 (2019). https://doi.org/10.1007/s40011-017-0959-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40011-017-0959-4