Abstract
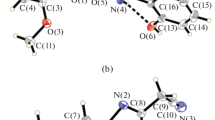
The oxidovanadium(IV) complex of composition [VO(acac)(SHA)] (where acac = [CH3COCHCOCH3−]); (SHA = [2-OHC6H4C(O)NHO−]) has been synthesized by the reaction of [VO(acac)2] with salicylhydroxamic acid (SH2A) in methanol + tetrahydrofuran and characterized by physicochemical and various spectral studies. The FTIR spectra indicated that salicylhydroxamate ion is bonded through carbonyl and hydroxamic oxygen (CONHO) atoms [O, O mode]. An optimized distorted square-pyramidal geometry around vanadium has been obtained by DFT/SIESTA using standard conjugate-gradient (CG) technique. From the HOMO–LUMO energy values, the molecular properties, viz. ionization potential (IP), electron affinity (EA), chemical potential (µ), hardness (η), softness (S), electronegativity (χ) and electrophilicity index (ω), have been calculated. The energy-resolved visualization of chemical bonding and molecular orbital contributions have been studied from the density of states, partial density of states and overall population density of states/crystal orbital overlap population and crystal orbital Hamiltonian population. The antibacterial activity of complex assayed against four bacterial strains, viz. E. coli, S. aureus, S. epidermidis and K. pneumoniae by MIC method, has shown it as promising growth-inhibiting agent.











Similar content being viewed by others
References
Crans DC, Smee JJ, Gaidamauskas E, Yang L (2004) The chemistry and biochemistry of vanadium and the biological activities exerted by vanadium compounds. Chem Rev 104:849–902
Etcheverry SB, Ferrer EG, Gonzalez-Baro AC, Parajon-Costa BS, Williams PAM (2009) Vanadis charms: from the mithology to the bioinorganic chemistry. J Argentine Chem Soc 97:127–150
Baran EJ (2000) Oxovanadium(IV) and oxovanadium(V) complexes relevant to biological systems. J Inorg Biochem 80:1–10
Thompson KH, Orvig C (2001) Coordination chemistry of vanadium in metallopharmaceutical candidate compounds. Coord Chem Rev 219–221:1033–1053
Monga V, Thomson KH, Yuen VG, Sharma V, Patrick BO, Macneill JH, Orvig C (2005) Vanadium complexes with mixed O, S anionic ligands derived from maltol: synthesis, characterization and biological studies. Inorg Chem 44:2678–2688
Molineuvo MS, Bario DA, Cortizo AM, Etcheverry SB (2004) Antitumoral properties of two new vanadyl(IV) complexes in osteoblasts in culture: role apoptosis and oxidative stress. Cancer Chemother Pharmacol 53:163–172
Karmaker S, Saha TK, Yoshikawa Y, Sakurai H (2010) Vanadyl-poly(glutamic acid) complexes as oral therapeutic agents for the treatment of type 1 like diabetic mice. Afri J Pharm Pharmacol 4:235–243
Kawabe K, Yoshikawa Y, Adachi Y, Sakurai H (2006) Possible mode of action for insuliunomimetic activity of vanadyl(IV) compounds in adipocytes. Life Sci 78:2860–2866
Nejo AA, Kolawole GA, Opoku AR, Muller C, Wolowska J (2009) Synthesis characterization and insulin enhancing studies unsymmetrical tetradentate schiff-base complexes of oxovanadium(IV). J Coord Chem 62:3411–3424
Sanna D, Micera G, Garribba E (2010) A quantitative study of the biotransformation of insulin-enhancing VO2+ compounds. J Biol Inorg Chem. 15: 825–839: new developments in the comprehension of the biotransformation and transport of insulin-enhancing vanadium compounds in the blood serum. Inorg Chem 49:174–187
Maurya MR (2003) Development of coordination chemistry of vanadium through bis(acetylacetonato)oxovanadium(IV): synthesis, reactivity and structural aspects. Coord Chem Rev 237:161–181
Pal D, Saha S (2012) Hydroxamic acid—a novel molecule for anticancer therapy. J Adv Pharm Technol Res 3(2):92–99
Muri EMF, Nieto MJ, Sindelar RD, Williamson JS (2002) Hydroxamic acids as pharmacological agents. Curr Med Chem 9:1631–1653
Codd R (2008) Traversing the coordination chemistry and chemical biology of hydroxamic acids. Coord Chem Rev 252:1387–1408
Ugwu DI, Ezema BE, Eze FU, Argoyu JI, Ezema CG, Ugwuja DI (2014) Synthesis and biological applications of hydroxamates. Am J Org Chem 4:26–51
Marimion CJ, Griffith D, Nolan KB (2004) Hydroxamic acids—an intriguing family of enzyme inhibitors and biomedical ligands. Eur J Inorg Chem 2004:3003–3016
Bell JH, Pratt RF (2002) Formation and structure of 1:1 complex between aryl hydroxamic acids and vanadate at neutral pH. Inorg Chem 41:2747–2753
Hall MD, Failes TW, Hibbs DE, Hambley TW (2002) Structural investigations of palladium(II) and platinum(II) complexes of salicylhydroxamic acid. Inorg Chem 41:1223–1228
Henderson W, Evans C, Nicholson BK, Facwcett J (2003) Coordination isomerism in salicylhydroxamate complexes of platinum(II) and palladium(II). Dalton Trans 13:2691–2697
Springer AL, Gall AS, Hughes KA, Kaiser RJ, Li G, Lund KP (2003) Salicylhydroxamic acid functionalized affinity membranes for specific immobilization of proteins and oligonucleotides. J Biomol Tech 14:183–190
Sharma N, Kumar V, Sharma R, Kumari M, Kanwar SS (2011) Coordination compounds of hydroxamatooxovanadium(IV) complexes with nitrogenous bases and their antimicrobial activities. Bull Chem Soc Jpn 84:855–861
Sharma N, Kanwar SS, Gupta R, Kumari L, Sharma R (2012) Reactions of bis(8-hydroxyquinolato)oxovanadium(IV) with hydroxamate ligands: a route providing mixed ligand and quinolinato free vanadium(IV) complexes. Bull Chem Soc Jpn 85:1310–1317
Sharma R, Sharma N (2012) Thermal studies of some biologically active oxovanadium(IV) complexes containing 8-hydroxyquinolinate and hydroxamate ligands. J Therm Anal Calorim 110:539–543
Sharma N, Kumari M, Sharma R (2012) Thermoanalytical studies of oxovanadium(IV) hydroxamate complexes. J Therm Anal Calorim 107:225–229
Sharma R, Sharma N (2013) A thermal behaviour and structural study of bis(hydroxamato)oxovanadium(IV) complexes. J Therm Anal Calorim 112:25–30
Soler JM, Artacho E, Gale JD, Garcia A, Junquera J, Ordejón P, Sánchez-Portal D (2002) The SIESTA method for ab initio order-N materials simulation. J Phys Condens Matter 14:2745–2779
Rowe RA, Jones MM (1957) Vanadium(IV) oxy(acetylacetonate). Inorg Synth 5:113–116
Troullier N, Martins JL (1991) Efficient pseudopotentials for plane wave calculations. Phys Rev B 43:1993–2006; Efficient pseudopotentials for plane-wave calculations: II. Operators for fast iterative diagonalization. Phys Rev B 43: 8861–8869
Kleiman L, Bylander DM (1982) Efficacious form for model pseudopotentials. Phys Rev Lett 48:1425–1428
Greenwood D, Slack R, Peutherer J, Barer M (2007) Medical microbiology: a guide to microbial infections: Pathogenesis, immunity, laboratory diagnosis and control, 17th edn. Churchill-Livingstone, Edinburgh
Mackie TJ, Collee JC, McCartney JE (1989) In: College JC, Dugluid JP, Frasor GA, Marmion BP (eds) Practical medical microbiology, 13th edn. Churchill-Livingstone
Geary WL (1971) The use of conductivity measurements in organic solvents for the characterization of co-ordination compounds. Coord Chem Rev 7:81–122
Figgis BN (1966) Introduction to ligand fields, 1st edn. Wiley Eastern Limited, NewDelhi
Ballhausen CJ, Grey HB (1962) The electronic structure of vanadyl ion. Inorg Chem 1:111–122
Fedorova EV, Rybakov VB, Senyavin VM, Anisimov AV, Aslanov LA (2003) Synthesis and structure of oxovanadium(IV) complexes [VO(acac)2] and [VO(sal: l-alanine) (H2O)]. Crystallogr Rep 50:224–229
Fischer DC, Barclay-Preet SJ, Balfe CA, Raymond KN (1989) Synthesis and characterization of vanadium(V) and -(IV) hydroxamate complexes: X-ray crystal structures of oxochloro bis(benzohydroxamato) vanadium(V) and oxoisopropoxo(N,N′-dihydroxy-N,N′-diisopropylheptanediamido)vanadium(V). Inorg Chem 28:4399–4406
Koopmans TA (1934) Zuordnung Über die, Wellenfunktionen von und Eigenwerten zu den Einzelnen Elektronen Eines Atoms. Physica 1:104–113
Pearson RG (2005) Chemical hardness and density functional theory. Chem Sci 117:369–377
Dronskowski R, Blöchl PE (1993) Crystal orbital Hamilton populations (COHP) energy-resolved visualization of chemical bonding in solids based on density-functional calculations. J Phys Chem 97:8617–8624
Acknowledgements
The authors would like to thank Department of science and Technology (DST), Government of India, New Delhi, for providing financial assistance for FTIR and UV–Vis spectrophotometer facility to the department under FIST program and Panjab University, Chandigarh, for recording mass spectra and elemental analyses. The authors thank Department of Biotechnology, Himachal Pradesh University, Shimla, for providing laboratory facilities.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is dedicated to the fond memory of our worthy teacher Late Prof. K.C. Malhotra, Ex Vice-Chancellor, H.P. University, Shimla.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sharma, N., Priya, B., Choudhary, V.K. et al. Potentially Antibacterial Mixed-Ligand Oxidovanadium(IV) Salicylhydroxamate Complex [VO(acac)SHA]: Synthesis, Characterization and Quantum Mechanical Study. Proc. Natl. Acad. Sci., India, Sect. A Phys. Sci. 90, 213–223 (2020). https://doi.org/10.1007/s40010-018-0577-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40010-018-0577-4