Abstract
Purpose
Up-to-date literature offers limited data about utilizing atorvastatin calcium (ATV) as a promising chondroprotective agent in osteoarthritis (OA). So, this study aims to develop a depot intra-articular (IA) delivery system for ATV to enhance its deposition in the articular joint.
Methods
A 33 D-optimal design was implemented to prepare ATV-loaded lecithin-coated zein nanoparticles. The optimized formulation (Opt-LCZN) was selected and imaged using a transmission electron microscope according to the desirability value. Various in-vitro and in-silico studies were conducted to evaluate the features of Opt-LCZN. Additionally, it was loaded into an injectable thermogel (Opt-LCZN-thermogel) and evaluated in-vivo in OA-induced Sprague Dawley rats.
Results
The Opt-LCZN showed entrapment efficiency of 70.00 ± 2.96%, particle size of 191.95 ± 17.42 nm, zeta potential of − 20.12 ± 0.79 mV, and polydispersity index of 0.25 ± 0.01. The docking studies revealed favorable binding of zein and ATV, confirmed by molecular dynamics simulation. The morphological examination displayed a bilayer spherical structure formed of a zein core enclosed by a lecithin coat. Furthermore, the formulated Opt-LCZN-thermogel achieved a remarkable sustained release profile, with nearly 50% of the drug being released over 144 h. Opt-LCZN-thermogel showed a significant reduction in inflammation in OA-induced rats, confirmed by knee joint swelling and knee bend test results, in addition to the pro-inflammatory and anti-inflammatory mediators’ levels. The protective effect of ATV can be markedly observed through histopathological examination.
Conclusion
Based on these outcomes, the formulated IA delivery system of ATV can be presented as an excellent candidate for ameliorating OA.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoarthritis (OA) is a widespread chronic crippling joint condition that primarily affects geriatrics. Joint inflammation is the root cause of OA as it contributes to initiating symptoms and disease progression (Liu-Bryan and Terkeltaub 2015). In OA, chondrocytes and synoviocytes release pro-inflammatory cytokines such as interleukin-6 (IL-6), interleukin-1ß (IL-1ß), and tumor necrosis factor-α (TNF-α) that upregulate the expression of several degrading enzymes as matrix-metalloproteinases (MMPs) (Robinson et al. 2016). Such catabolic factors are responsible for the progressive destruction of articular cartilage and remodeling of the subchondral bone, which are the hallmarks of the disease (Robinson et al. 2016). Hence, affected individuals experience a significant decline in their quality of life as OA can ultimately cause joint pain, edema, stiffness, restricted movement, disability, or even morbidity (Jansen and Mastbergen 2022). Currently, the available treatments for OA are used for the symptomatic relief of pain rather than managing its underlying cause, the most common of which are paracetamol and non-steroidal anti-inflammatory drugs. However, their prolonged use can cause deleterious effects on the kidneys, cardiovascular system, gastrointestinal tract, and other major organs (Bindu et al. 2020). As the prevalence of OA continues to rise, there is an urgent need to utilize an alternative therapy technique with diminutive side effects that can reduce the progression of OA by targeting inflammation therapeutically and protecting the articular cartilage during the disease.
Atorvastatin calcium (ATV) is an anti-hyperlipidemic drug that exerts its action through the competitive inhibition of hydroxy-2-methyl-glutaryl coenzyme A (HMG-CoA) reductase. Several studies reported that ATV possesses anti-inflammatory and analgesic effects by inhibiting chemokines and neutrophil infiltration (Barsante et al. 2005; Dwajani et al. 2012; Bhattacharjee et al. 2017). In addition, it has recently been acknowledged to possess immunomodulatory and chondroprotective effects, which were assumed to occur on two fronts: first, by direct blockage of MMP-13, and second, by indirect inhibition of the synthesis and the release of MMPs by chondrocytes through inhibiting the inflammatory cytokines (Pathak et al. 2015; Parsamanesh et al. 2019). Such effects qualify it to be used as a valuable agent to control the symptoms of OA and halt disease progression. However, the poor oral bioavailability (⁓12%) of ATV resulting from its extensive first-pass metabolism, crystalline nature, in addition to its diminished aqueous solubility (0.1 mg/ml) limits its therapeutic utility in OA (Choudhary et al. 2012). Its poor performance requires excessive dose administration, which may harmfully affect the liver and kidneys and may cause rhabdomyolysis and arthralgia (Ronaldson et al. 2006). To overcome these restrictions, site-specific delivery of ATV through the intra-articular (IA) route may enhance its bioavailability, reduce its systemic adverse effects, and directly deliver the drug to the target site (Jones et al. 2019).
Zein is an emerging high molecular weight prolamine protein extracted from corn kernels. It is formed from numerous hydrophobic amino acids and a limited amount of hydrophilic amino acids (Shukla and Cheryan 2001). It can self-assemble, forming spherical nanoparticles by applying the antisolvent precipitation technique; hence, many attempts have investigated the possibility of using it as a suitable delivery platform for various therapeutic agents such as curcumin, lutein, gallic acid, and resveratrol (Hu et al. 2012; Radwan et al. 2020; Khan et al. 2021; Zhang et al. 2021a). However, the limited colloidal stability of zein stood as an obstacle to its formulation as a stable delivery system; this urged the researchers to investigate the use of different stabilizing techniques, such as the application of the surface coating and the addition of various stabilizers (Chuacharoen and Sabliov 2016; Khan et al. 2019; Radwan et al. 2020). In addition to the capability of zein to form sustained release delivery systems with high encapsulation efficiency, it also has the advantage of being non-toxic, economical, biodegradable, and biocompatible (Chuacharoen and Sabliov 2016; Zhang et al. 2021a). These multitudes of properties nominate zein as a promising delivery system in OA.
Thermogel is a polymeric thermoresponsive system that can remain in solution form at ambient temperature while forming a depot gel upon contact with the target site at physiological temperature (Zhang et al. 2015). It has attracted the attention of several investigators to be applied to the administration of drugs through the IA route as it can act as a depot delivery system that prolongs the drug residence by limiting its clearance at the knee joint, hence minimizing the frequency of administration (Mok et al. 2020). Due to its high safety profile and hydrosoluble properties, Poloxamer 407 is commonly used as a thermoresponsive polymer. However, its diminished mechanical property and limited residence time at the target site necessitate the addition of a mucoadhesive polymer that would serve as a crosslinker, facilitating the tight packing of the poloxamer micelles above the sol-to-gel transition temperature (Jung et al. 2017). In recent years, hyaluronic acid (HA) has gained much attention for being used as a natural mucoadhesive polymer for its favorable anti-inflammatory action in addition to its nonimmunogenic, biocompatible, and biodegradable properties (Mayol et al. 2008). In the arthritic joints, HA concentration is altered and declined by 33–50%, restricting its functionality in preserving normal joint biomechanics (Watterson and Esdaile 2000). So, administering exogenous HA would maintain joint lubrication, minimize friction, and restore articular viscoelasticity in OA.
Accordingly, the present study aims to formulate a novel depot IA delivery system to boost the drug residence time at the affected joint and deliver ATV to the target site to alleviate arthritic inflammation. This is accomplished by encapsulating ATV into zein nanoparticles comprised of eugenol and stabilized with a lecithin coat and the use of Gelucire® 44/14 as a surfactant. The optimized formulation with the highest desirability value is generated using a 33 D-optimal design. It is further incorporated into a thermogel to prolong the drug action and produce a depot effect. This final formulation is physiochemically assessed in-vitro and its therapeutic action is assessed in-vivo on Sprague Dawley rats.
Materials and methods
Materials
Atorvastatin calcium (ATV) was kindly donated by Misr Company, Cairo, Egypt. Zein protein (molecular weight 22–27 KDa) was obtained from Acros Organics Co., Geel, Belgium. Lecithin from egg yolk, Eugenol, Poloxamer 407, complete Freund’s adjuvant (CFA), and cellulose membrane (12–14 KDa molecular weight cutoff) was gained from Sigma-Aldrich Corp., St. Louis, MO, USA. Hyaluronic acid (HA) (Mwt = ~ 600–750 kDa) was supplied by Shanghai Jiaoyuan Industry Co., Ltd., China. Gelucire® 44/14 was gifted by Gattefosse, Saint-Priest, France. Methanol, Ethanol 95%, and Formaldehyde were obtained from El Nasr Company, Cairo, Egypt.
Methods
Fabrication of ATV-loaded lecithin-coated zein nanoparticles (LCZN)
The ethanol-injection technique was employed to synthesize ATV-loaded LCZN efficiently. In brief, the organic phase was prepared by dissolving lecithin, zein, eugenol (50 mg), and ATV (10 mg) in 2.5 ml ethanol (95% v/v) in a sealed flask using a magnetic stirrer (MS-300HS, Misung Scientific Co., Korea) with continuous stirring at 1500 rpm at ambient temperature. The aqueous phase was prepared by dissolving Gelucire® 44/14 as an emulsifying agent in deionized water. Afterwards, the organic phase was injected dropwise into the fourfold greater vehicle of the aqueous phase, stirring at 1500 rpm at ambient temperature. Then, the prepared formulation was subjected to continuous sonication for 10 min using a probe sonicator (ChromTech Model UP-500, 220V). Finally, for solvent evaporation, the formulation was left to stir at 1500 rpm at ambient temperature till the complete removal of the ethanolic residues. The final formulation was stored overnight at 4 °C for further investigations.
Statistical design construction
A 33 D-optimal design was employed to investigate the effect of 3 independent variables, each at 3 levels, on the fabrication of LCZN formulations via Design-Expert® software version 13 (Stat-Ease, Inc., Minneapolis, MN, USA). The design claimed to construct a total of 16 runs. The independent variables were lecithin amount (X1), zein amount (X2), and Gelucire® 44/14 concentration (X3), while other formulation variables remained constant. The dependent variables studied were entrapment efficiency percentage (EE%) (Y1), particle size (PS) (Y2), polydispersity index (PDI) (Y3), and zeta potential (ZP) (Y4). Table 1 depicts the investigated independent variables with the actual values of their levels, while Table 2 exhibits the resulting formulation trials with their detailed characterizations.
Characterization and optimization of ATV-loaded LCZN
Drug content determination
An aliquot of each formulation was transferred to a 10 ml volumetric flask and diluted in methanol. Then, a UV spectrophotometer (Shimadzu UV1650, Spectrophotometer, Kyoto, Japan) was employed to measure the UV absorbance of the diluted sample at λmax 244 nm (Elgendy et al. 2023). The following equation was applied to assess the drug content, and all measurements were carried out in triplicates (El-Leithy et al. 2018):
Measurement of entrapment efficiency (EE%)
The amount of drug entrapped into the nanoparticles was estimated by direct centrifugation of 1 ml of the prepared formulation utilizing a cooling centrifuge (Sigma 3 K 30, Germany) at 15,000 rpm for 2 h at 4 °C (El Assasy et al. 2019). First, the supernatant was disposed of, and then lysis of the formed residue was performed using methanol until a clear solution was obtained. Finally, the clear solution was analyzed spectrophotometrically at λmax 244 nm, and each formulation was determined in triplicates to directly estimate the entrapped drug using the following equation (Abdellatif et al. 2020):
Assessment of particle size (PS), polydispersity index (PDI), and zeta potential (ZP)
Before measuring PS, PDI, and ZP, an adequate dilution of the freshly prepared formulations was performed using deionized water until a slightly opalescent appearance was achieved. A Zetasizer (Malvern Instruments, Malvern, UK) was used to measure the formed nanoparticles’ mean diameter and size distribution at 25 °C based on examining the oscillations in scattered light intensity produced by the Brownian motion of the dispersed particles. Additionally, the ZP was assessed using the same instrument by directly injecting the diluted samples into the Zeta particle sizer capillary cell with electrodes on both sides to determine the electrophoretic motion of the particles within the electric field. All measurements were performed three times to guarantee the accuracy of the readings.
Validation and optimization
Validation of the proposed models was tested by calculating 95% two-sided prediction intervals for the predicted values of responses. The values measured for all studied responses for all runs performed were evaluated to determine whether they lie within the 95% prediction interval (Fouda et al. 2018). Statistical optimization was performed by maximizing EE% and the absolute value of ZP while minimizing PS and PDI to select the optimized lecithin-coated zein nanoparticle formulation (Opt-LCZN). The selection of this formulation was based on the desirability value, which permits the examination of all constraints concurrently. So, the formulation exhibiting the highest desirability function was selected as Opt-LCZN. Finally, this statistically suggested formulation was prepared and characterized. Then, a comparison of the actual and predicted values of the responses from the model was carried out by computing the bias percentage equation to assure the accuracy of the model performance as follows (Younes et al. 2018):
Assessment of the in-vitro release
The dialysis bag diffusion method was implemented to study the drug release rate from Opt-LCZN and ATV solution (1 mg/ml). Initially, the pre-immersed dialysis bags were filled with an aliquot of either Opt-LCZN or ATV solution containing equivalent amounts of the drug (1 mg) and firmly clamped at both ends. The formerly prepared dialysis bags were immersed in tightly closed bottles containing 50 ml of the release medium (90% PBS 7.4 + 10% ethanol) (Kamel et al. 2016). Afterwards, these bottles were placed in a thermostatically controlled water bath shaker (Memmert, Munich, Germany) adjusted at 37 ± 0.5 °C and a constant speed of 100 rpm. A sample of 3 ml was withdrawn at various time intervals (0.5, 1, 2, 4, 6, 8, 10, 12, 24, 30, 48, 54, 72, 78, 96, 102, and 144 h) and was replenished with fresh release medium. Finally, the withdrawn samples were measured spectrophotometrically at λmax 244 nm. The experiment was conducted in triplicates to ensure accurate results.
In-silico studies
Docking studies
The molecular operating environment (MOE) software version 2019.0102 (Chemical Computing Group, Montreal, QC, Canada) was employed to conduct the various docking assessments (Vilar et al. 2008). First, the compounds were drawn in 2D in ChemDraw 20.1.1 before being exported to 3D in MOE. Using the UniProt ID: Q41877, the zein protein structure was attained from the AlphaFold Protein Structure Database (ebi.ac.uk). Then the energies of each molecule forming the Opt-LCZN, namely ATV, lecithin, zein, Gelucire® 44/14 (mixture of lauroyl polyoxyl-32 glycerides and lauroyl macrogol-32 glycerides), and eugenol were diminished using AMBER10:EHT (extended Huckel theory) before initiating the docking. Afterwards, each component was evaluated as a possible receptor for ATV binding. Finally, a high-precision docking study was carried out using an induced fit technique and a triangle matcher as a scoring function (Albash et al. 2022). The docking outcomes were investigated based on both molecular interactions and docking scores.
Molecular dynamics simulation (MDS)
The MOE and nanoscale molecular dynamics (NAMD) 2.1.1 software were implemented to perform all MDS calculations (Phillips et al. 2005). Three MDS experiments were conducted on free ATV, free zein protein, and ATV-zein complex to validate the docking evaluation results. MOE was used to set up the Langevin dynamics, solvation, pressure, temperature and periodic boundary conditions for NAMD 2.1.1. The energy of all three systems was minimized using the steep descent minimization technique in AMBER10:EHT until 0.1 RMS kcal/mole/A2 was achieved (Case et al. 2005; El-Hasab et al. 2017; El-Hassab et al. 2020). The energy-minimized structures were equilibrated for 5 ns. The particle mesh Ewald (PME) technique, with a cutoff of 12 Å, was applied to determine the long-range electrostatic value. The three equilibrated systems went into production with no constraints for 150 ns at a time step of 2 fs, with structural coordinates stored at 100 ps intervals. The root mean square deviation (RMSD) of all heavy atoms in the system was computed using the measured trajectories from the production phase.
Morphological examination
The morphological features of the Opt-LCZN were visualized with the aid of a transmission electron microscope (TEM) (Joel JEM 1230, Tokyo, Japan) operating at 80 kV. Before the microscopic examination, a drop of the adequately diluted sample was negatively stained using 2% w/v phosphotungstic acid solution. It was left to dry for about 10 min above a carbon-coated copper grid.
Thermal analysis using differential scanning calorimetry (DSC) technique
Approximately 5 mg of pure ATV, lecithin, zein, Gelucire® 44/14, eugenol, the physical mixture of ATV along with the other constituents, and the lyophilized Opt-LCZN were thermally investigated with the assistance of a differential scanning calorimeter (Shimadzu, DSC-60, Japan) calibrated by employing purified indium as the standard reference. Samples were tested in a constant heat range from 25 to 300 °C on an aluminum pan with a 25 ml/min flow of inert nitrogen and a scanning rate of 10 °C/min.
Fourier-transform infrared (FTIR) spectroscopy
The spectra of pure ATV, lecithin, zein, Gelucire® 44/14, eugenol, the physical mixture of ATV together with the other components, and the lyophilized Opt-LCZN were analyzed using FTIR spectroscopy (Jasco, FT/IR 6100, Japan). Concomitant mixing of about 2–3 mg of each sample with dry KBr was carried out which in turn was subjected to compression into a disk under pressure at ambient temperature. Then the disc was scanned at a wavelength range of 400–4000 cm-1.
Investigating the effect of aging
The Opt-LCZN was refrigerated at 4 °C in completely sealed vials to determine whether storage would affect the various formulation parameters. The stored formulation was examined during the storage period for any agglomeration or phase separation. After 30 and 90 days of storage, the previously measured responses (EE%, PS, PDI, and ZP) were statistically compared with the values of the freshly prepared formulation by applying Student’s t-test using SPSS® software 22.0. While the effect of storage on the release profile of the stored formulation was determined by calculating the similarity factor (ƒ2) according to the following equation (El-Helaly et al. 2017):
where n is the total sampling time, Rt and Tt are the average percentage of drug released from the fresh and stored samples at time t. An ƒ2 value ranging from 50 to 100 indicates the similarity of the release profiles.
Preparation of Opt-LCZN loaded thermogel (Opt-LCZN-thermogel)
The Opt-LCZN-thermogel was fabricated by dissolving a fixed concentration of poloxamer 407 (15% w/v) in an accurately measured volume of Opt-LCZN (equivalent to 1 mg/ml) at 4 °C. This solution was stirred at 1500 rpm using a magnetic stirrer for 1 h. Following that, an amount of HA equivalent to 0.2% w/v was added to the former solution, and then it was left to stir again to guarantee adequate solubility of the added solutes within the formulation.
Characterization of the Opt-LCZN-thermogel
Sol–gel transition temperature (T sol-gel) measurement
The Tsol-gel was determined by a visual inspection technique that used a hot plate magnetic stirrer (Abd Ellah et al. 2019). About five milliliters of the prepared Opt-LCZN-thermogel, previously maintained at 4 °C, was added to a beaker with a thermometer immersed and continuously stirred at 30 rpm. A gradual increase in temperature was performed, and the temperature at which the magnet ceased its rotation was reflected in the Tsol-gel, which was recorded in triplicates to confirm accurate measurement.
Determination of gelation time
The gelation time was evaluated by employing the test tube tilting technique. About 2 ml of the Opt-LCZN-thermogel were placed in a test tube and heated in a water bath at the predetermined Tsol-gel. Afterwards, the test tube was tilted at 90 °C to notice the flowability of the gel. The gelation time is the time taken for solidifying the Opt-LCZN-thermogel, recorded in triplicates (Morsi et al. 2016).
Viscosity evaluation and rheological study
A cone and plate viscometer (DV-III Programmable Brookfield Rheometer, Stoughton, MA) with thermostatically controlled water circulation was operated to confirm the conversion of the Opt-LCZN-thermogel into a solidified gel at the predetermined Tsol-gel. The viscosity was measured at 25 °C, then the Opt-LCZN-thermogel was allowed to solidify and was remeasured again at 35 °C at 10 rpm. Moreover, the rheological behavior of the solidified Opt-LCZN-thermogel was determined at multiple shear rates (10, 20, 30, 40, and 50 rpm) with 10 s between each two successive angular velocities using spindle 52 targeting torque values exceeding 10% (Morsi et al. 2016). All measurements were performed in triplicates. The pattern of flow of the Opt-LCZN-thermogel was generated by plotting the shear stress (x-axis) against the shear rate (y-axis).
Syringeability
The passage through the syringe was examined by inserting about 1 ml of the Opt-LCZN-thermogel in a 5 ml plastic syringe with a 21-gauge needle at ambient temperature. It is believed to be syringeable when it succeeds in passing easily through the needle. The experiment was conducted in triplicate (Hanafy and El-Ganainy 2020).
Assessment of the release rate of ATV from the Opt-LCZN-thermogel
The dialysis diffusion method was applied to evaluate the release of ATV from the Opt-LCZN-thermogel in the same manner as stated before. An amount equivalent to 1 mg ATV was placed in the dialysis bag. The withdrawn aliquots (3 ml) at pre-decided time intervals (0.5, 1, 2, 4, 6, 8, 10, 12, 24, 30, 48, 54, 72, 78, 96, 102, and 144 h) were replenished with fresh medium to maintain a constant volume. Then, they were spectrophotometrically measured at λmax 244 against a similarly treated blank. The experiment was repeated in triplicates. Statistical difference between ATV release from the Opt-LCZN, ATV-thermogel, and the Opt-LCZN-thermogel was performed using univariate followed by Tukey’s post-hoc test using SPSS® software 22.0. Statistical significance was set at p ≤ 0.05. Furthermore, the release profiles were further examined by fitting the acquired data into various model equations to assess the release mechanism from various systems using the DDSolver software (Excel Add-in). Based on the highest correlation coefficient (R2), the best-fit model was selected (Sokol et al. 2022).
Terminal sterilization
The Opt-LCZN-thermogel was terminally sterilized by gamma irradiation (60Co irradiator) (National Center for Radiation Research and Technology, Cairo, Egypt) operating at a dose of 10 kGy (1.19 kGy/hr). It was first packed inside a polyurethane container, which, in turn, was placed in ice to prevent any particle aggregation or melting that might be caused by increasing temperature during the sterilization process. The similarity factor (ƒ2) was calculated to determine whether the sterilization process affected the rate of drug release from the Opt-LCZN-thermogel or not via the same equation that was previously described in the section “Investigating the effect of aging”.
In-vivo study
Animals
Twenty-four adult Sprague Dawley male rats weighing between 160 ± 20 g (age: 8–10 weeks) were housed in cages of appropriate sizes (maximum of 3 animals per cage) to guarantee the well-being of the animals. They were acclimatized for two weeks under standard laboratory conditions of 28 ± 2 °C, 55 ± 5% relative humidity, interchanging 12 h light and dark cycles, and had unrestricted access to their standard diet pellets and water. All animal experiments complied with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and adhered to ARRIVE guidelines (National Research Council 2011). The experimental protocol was approved by the Research Ethics Committee, Faculty of Pharmacy, Cairo University, Egypt (Ethical approval no.: PI 2947). The animals were randomly distributed among the experimental groups, ensuring the weights of rats in all groups were comparable. All the tests were carried out by operators blinded to the groups. At the end of the experiment, blood samples were collected from the rats’ retro-orbital plexus and then they were euthanized for further histopathological examination.
Experimental design
On days 1 and 7 of the study, arthritis was induced by an IA injection of 0.5 ml of ovalbumin (5 mg/ml) in complete Freund’s adjuvant (CFA) in the right knee of each rat (Kamel et al. 2016; Chang et al. 2021; Alazragi and Baeissa 2023). The left knee was kept un-injected as a control. Treatment started on the 14th day of induction based on the development of symptoms and was extended for two weeks. Rats were randomly assigned into four groups (n = 6) as follows:
-
Control group the negative control group representing healthy normal rats;
-
CFA group the positive control group representing the arthritic untreated rats;
-
ATV-Opt-LCZN-thermogel group the arthritic rats treated with an IA injection of ATV-Opt-LCZN-thermogel at a dose of 0.4 mg/ml/kg on days 14 and 21 (Dinc et al. 2012);
-
ATV-thermogel group the arthritic rats treated with an IA injection of ATV-thermogel at a dose of 0.4 mg/ml/kg on days 14 and 21 (Dinc et al. 2012).
Evaluation of osteoarthritis induction
The rats were weighed and monitored for signs of arthritis from day 1 to day 14. The severity and pathological changes in the knee joint were observed and the scoring index for arthritic manifestations was calculated. The severity of symptoms in each rat was assessed by grading the knee joint on a scale of 0–3, taking into account the variations in erythema, edema, presence of nodules, and the involvement of other non-injected joints. The scoring criteria were as follows: 0 indicated no erythema, swelling, or nodules; 1 indicated erythema with mild swelling but no nodules; 2 indicated erythema with moderate swelling, with or without nodules; and 3 indicated erythema with severe swelling that limited overall movement, accompanied by the presence of nodules or lesions (Mossiat et al. 2015). Rats with an arthritic score of more than 4 were considered osteoarthritic rats (El‑Tedawy et al. 2020) and were randomized into different experimental groups. The induction rate in our model was 83.3%.
Evaluation of knee joint swelling
Rat knee joint diameter was measured using a vernier caliper (ColeParmer Instrument Co.) at several intervals (days 1, 7, 14, 21, and 28). At each time point, the mean value of the two perpendicular diameters of the joint was determined. In addition, the percentage change in knee swelling was measured and compared to the control left knee.
Behavioral testing: knee bend test
The knee bend test entails counting the number of squeaks or struggle reactions in response to five alternate knee flexions and extensions conducted within the physiological limits of knee flexion/extension. The response to each joint movement determined the test score, which was as follows: 0 no response to any joint movement; 0.5 represents a struggle to maximal flexion/extension; 1 represents a struggle to moderate flexion/extension or vocalizations to maximal flexion/extension; 2 represents vocalizations to moderate flexion/extension.
Blood sampling
On day 28, blood samples were withdrawn from the retro-orbital plexus and allowed to clot. The blood samples from different study groups were then centrifuged at 4000 rpm for 10 min, and the sera were collected. Sera were kept at − 80 °C for subsequent estimation of pro-inflammatory and anti-inflammatory mediators’ levels.
Assessment of pro-inflammatory mediators in serum
The serum levels of inflammatory mediators (IL-1ß, IL-6, and TNF-α) were assessed by using Enzyme-linked immune sorbent assay (ELISA) kits developed by Thermo Fisher Scientific for assessing IL-1ß (Catalog# BMS630), Cloud-Clone Corp for assessing IL-6 (Catalog# SEA079Ra) and TNF-α (Catalog# KRC3011C). All procedures were executed precisely according to the manufacturer’s guidelines.
Assessment of anti-inflammatory mediators in serum
The serum levels of IL-4, IL-10, and IL-13 were assessed using ELISA kits. IL-4 and IL-10 were measured using kits developed by Thermo Fisher Scientific (Catalog# BMS628 and ERA23RB), respectively. An ELISA kit developed by Boster Biological Technology, Pleasanton, CA, USA (Catalog# ABIN6957027). All procedures were executed exactly according to the manufacturer's guidelines.
Histopathological examination
On day 28, rats were anesthetized with ketamine (75 mg/kg; i.p.) and euthanized, and the knee joints were then separated and processed for light microscopical examination. After being fixated in 10% formalin for 24 h, the samples were decalcified in 5% w/v formic acid for 72 h, followed by washing with tap water. Then, they were subjected to a series of alcohol dilutions to remove excess moisture. Afterwards, specimens were cleared in xylene and then immersed in paraffin at 56 °C in a hot water oven for 24 h. The slide microtome prepared Paraffin beeswax tissue blocks for sectioning at 4 μm thickness. Then, the specimens were stained with hematoxylin and eosin (H and E) stain and imaged by a Full HD microscopic imaging system (Leica Microsystems GmbH, Wetzlar, Germany) (Mohamed et al. 2022).
Statistical analysis
Data were displayed as mean ± S.D. GraphPad Prism 7 software was used to statistically analyze the data by applying the one-way and two-way ANOVA with Tukey’s multiple comparisons test for pairwise comparisons. Values were considered significant when p ≤ 0.05.
Results and discussion
Fabrication of ATV-loaded lecithin-coated zein nanoparticles (LCZNs)
A major challenge was when preparing zein nanoparticles due to their poor water solubility and low colloidal stability. This led to particle aggregate formation and precipitation, hence losing their functionality and limiting their application in drug delivery (Pascoli et al. 2018). To surmount this decreased stability, dual stabilization of zein nanoparticles was carried out by adding surfactant and applying a stabilizing surface coating. Gelucire® 44/14 was added as a PEGylated surfactant having an HLB value of 14, indicating good water solubility with the capability of solubilizing hydrophobic agents, thus enhancing the colloidal stability of zein nanoparticles (Kawakami et al. 2004). Lecithin, a phospholipid food emulsifier, is composed of phosphatidylcholine being the hydrophilic head in addition to phosphatidylethanolamine and phosphatidylinositol being the two hydrophobic tails (Chuacharoen and Sabliov 2016). Hence, lecithin was applied as a coat surrounding the surface of zein nanoparticles via hydrogen bonds, hydrophobic effects, and electrostatic interactions between the hydrophilic and hydrophobic portions of zein with the polar heads and the alkyl chains of lecithin (Gagliardi et al. 2021). Furthermore, the unique structure of lecithin qualified it to exert an additional emulsification action by interacting simultaneously with both the hydrophilic and hydrophobic ingredients (Chuacharoen and Sabliov 2016). Consequently, zein nanoparticles were successfully synthesized by the dual solubilization and stabilization action of both Gelucire® 44/14 and lecithin together with the surface coating of zein nanoparticles using lecithin. Moreover, eugenol was incorporated during the preparation of the formulation to enhance the solubilization of the lipophilic drug and hence allow its encapsulation within the polymeric core (El-Nabarawi et al. 2018).
Characterization and optimization of ATV-loaded LCZN
Determination of the drug content
As represented in Table 2, the drug content of LCZN ranged from 86.71 ± 2.07 to 114.36 ± 0.88% and was in accordance with the acceptable range stated by the British Pharmacopeia (85% to 115%).
Entrapment efficiency (EE%)
As represented in Table 2, the EE% of LCZN ranged from 15.11 ± 2.82 to 70.00 ± 2.96% which exhibited a linear model with a non-significant lack of fit (p = 0.5896). The following equation represents the polynomial model for EE% (Y1):
Increasing the lecithin amount (X1) significantly increased EE% (p < 0.0001) as illustrated in Fig. 1a-I. Lecithin formed a lipophilic coat surrounding the polymeric zein core. Hence, introducing more lecithin thickened this coat which acted as a barrier that hindered the escape of the drug from the core (Hamdi et al. 2020). These findings were consistent with those observed by Hamdi et al., who found that increasing the lecithin shell hindered drug diffusion from the PLGA core.
Increasing the zein amount (X2) significantly increased EE% (p = 0.0181) as illustrated in Fig. 1a-II. This could be attributed to the distinctive structure of zein polymers, mainly composed of hydrophobic amino acids such as leucine, proline, and alanine that can aggregate to form polymeric nanoparticles. So, adding more zein might have resulted in a larger amount of drug entangled in the polymeric matrix (Ahmed 2018).
Increasing Gelucire® 44/14 concentration (X3) significantly decreased EE% (p < 0.0001), as illustrated in Fig. 1a-III. Its increase might have increased the solubilization and, hence, the drug diffusion from the polymeric core of zein nanoparticles to the external aqueous phase (Elsewedy et al. 2020). Moreover, the interaction between lecithin amount and zein amount (X1X2) resulted in a significant negative influence on EE% (p = 0.0256), as illustrated in Fig. 1a-IV.
Particle size (PS)
As represented in Table 2, the PS of the LCZN ranged from 191.95 ± 17.42 to 994.86 ± 7.07 nm, which indicates that all prepared formulations were in the nanometric range. This response fits a 2FI model with a non-significant lack of fit (p = 0.5378). The following equation represents the polynomial model for PS (Y2):
Although increasing the lecithin amount (X1) increased EE%, surprisingly its increase resulted in the formation of smaller particles (p < 0.0001) as illustrated in Fig. 1b-I. Localization of lecithin on the surface of the zein core might have lowered the interfacial free energy, allowing for the efficient subdivision of the formed nanoparticles, and resulting in reduced PS (Aboumanei and Mahmoud 2020).
Increasing the zein amount (X2) significantly increased PS (p < 0.0001) as illustrated in Fig. 1b-II. The elevation of the amount of zein in the organic phase raised the viscosity of the formulation, hindering the formation of smaller particles during sonication (El-Lakany et al. 2018). These findings were consistent with that reported by El-Lakany et al. where raising the zein concentration formed larger particles with higher EE%.
Increasing Gelucire® 44/14 concentration (X3) produced nanoparticles of larger size (p < 0.0001), as illustrated in Fig. 1b-III. Adding more surfactant molecules might have accumulated within the polymeric core and on the surface of the nanoparticles, which resulted in the expansion of the dense structure of the nanoparticles, forming huge particles (Salama et al. 2020).
Interaction between lecithin amount and zein amount (X1X2) resulted in a significant positive influence on PS (p < 0.0001), as illustrated in Fig. 1b-IV. Conversely, the interaction between lecithin amount and Gelucire® 44/14 concentration (X1X3) resulted in a significant negative influence on PS (p < 0.0001), as illustrated in Fig. 1b-V. Moreover, the interaction between zein amount and Gelucire® 44/14 concentration (X2X3) resulted in a significant negative influence on PS (p < 0.0001), as illustrated in Fig. 1b-VI.
Polydispersity index (PDI)
As represented in Table 2, the PDI of the LCZN ranged from 0.25 ± 0.01 to 0.44 ± 0.02, indicating that the size distribution of the prepared formulations was mainly monodispersed. This model fits a linear model with a non-significant lack of fit (p = 0.9261). The following equation represents the polynomial model for PDI (Y3):
Increasing the lecithin amount (X1) resulted in the formation of more uniform particles (p < 0.0001), as illustrated in Fig. 1c-I, which might be related to its effect on the PS. The presence of lecithin on the surface of zein nanoparticles acted as a mechanical barrier that prevented the coalescence of the particles and modified their size distribution. These findings were in accordance with that observed by Dai et al., who reported that raising the lecithin amount decreased the PDI of zein nanoparticles (Dai et al. 2016). On the other hand, the amount of zein (X2) had a non-significant influence on PDI (p = 0.3620).
Increasing Gelucire® 44/14 concentration (X3) significantly increased PDI (p < 0.0001), as illustrated in Fig. 1c-II. As previously discussed, increasing Gelucire® 44/14 concentration enhanced its accumulation within the polymeric core and above the polymeric surface. This formed asymmetrical larger particles with a wide range of dimensional distribution. This result agreed with that previously reported by Younes et al., where adding more surfactant molecules to the formulation resulted in enlarged particles with uneven particle size distribution (Younes and Habib 2022). Also, the interaction between lecithin amount and Gelucire® 44/14 concentration (X1X3) resulted in a significant negative influence on PDI (p = 0.0235) as illustrated in Fig. 1c-III.
Zeta potential (ZP)
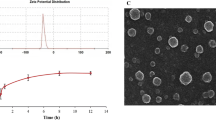
The ZP values reflect the net surface charge on the nanoparticles and evaluate the stability of the formulations. Elevated values of the ZP, irrespective of the positive or negative sign, indicate better stability due to the increased electrostatic repulsive forces between the adjacent particles, preventing cluster formation and flocculation. As represented in Table 2, the ZP values of the LCZNs ranged from − 7.23 ± 0.43 to − 20.12 ± 0.79 mV. This response exhibited a reduced quadratic model with a non-significant lack of fit (p = 0.9996). The following equation represents the polynomial model for ZP (Y4):
Increasing the lecithin amount (X1) significantly increased ZP (p < 0.0001), as illustrated in Fig. 1d-I, due to the increase in the negatively charged phosphate group existing in the lecithin structure. So, the net negative charge of the nanoparticles was raised (Dai et al. 2017).
Increasing the zein amount (X2) significantly decreased ZP (p = 0.0001), as illustrated in Fig. 1d-II. Adding more zein in the formulations increased the number of positively charged amino groups in their structure, leading to a decreased net negative charge found on the particles (Souzandeh and Netravali 2019).
Increasing Gelucire® 44/14 concentration (X3) resulted in a significant quadratic decrease in ZP (p = 0.0018), as illustrated in Fig. 1d-III. Gelucire® 44/14 is a mixture of 30% glycerol ester and 70% PEG-ester with fatty acids (Hathout and Woodman 2012). So, its addition to the formulation might have raised the amount of PEG moieties, characterized by the high shielding properties that mask the negative charge imparted by the lecithin coat (El-Gogary et al. 2014).
Validation and optimization
The D-optimal design was implemented to produce effect testing estimates with the least standard errors, as it optimizes the information in the selected set of experimental runs about the stated model (Krtalić et al. 2018). The responses evaluated for all the tested formulations appeared within the 95% prediction interval. Hence, the design adequately estimated the actual values of the responses. With the assistance of Design expert® software, the optimized formulation (Opt-LCZN) was selected according to the previously listed constraints in Table 1 and showed a maximum desirability of (0.980). The Opt-LCZN was composed of lecithin amount (X1) of 75 mg, zein amount (X2) of 25 mg, and Gelucire® 44/14 concentration (X3) of 2%; this corresponds to a formerly prepared formulation (F15) whose response values were already represented in Table 2. Based on the bias percentage calculation, all the measured actual values of the Opt-LCZN showed less than a 5% difference from the predicted values, indicating the validity of the statistical design and confirming its prediction capability within data uncertainty (Fouda et al. 2018). The actual and predicted values of the Opt-LCZN are stated in Table 1.
Assessment of the in-vitro release
As illustrated in Fig. 2, the drug release was more delayed by the Opt-LCZN than by the ATV solution, which ultimately released the drug within 4 h. The Opt-LCZN followed a biphasic release behavior with an initial burst effect followed by a sustained release pattern. The initial burst effect might be due to the presence of free drug on the surface of the nanoparticles (Li et al. 2013). Further sustainment of the drug might be attributed to slow water diffusion into the hydrophobic zein core, resulting in decreased hydrolysis of the nanoparticles and controlled release of the encapsulated drug. These findings were similar to those obtained by Dong et al., where the release of doxorubicin from zein nanoparticles was controlled for a few days (Dong et al. 2016).
In-silico studies
Docking studies
Each component forming the optimized formulation was considered a possible receptor for ATV to determine their potential interactions with the drug. ATV could promote favorable binding to each component of the formulation. It attained binding scores of − 5.3, − 3.2, − 3.9, − 3.8, and − 11.4 kcal/mole with lecithin, eugenol, lauroyl polyoxyl-32 glycerides, lauroyl macrogol-32 glycerides and zein, respectively. As illustrated in Fig. 3 (I), ATV was involved in various interactions with the five components, particularly the zein protein. The docking outcomes highlighted the efficient binding of zein to the drug, which called for further confirmation using molecular dynamics simulations.
Molecular dynamics simulation (MDS)
Several studies into drug discovery utilized the MDS technique to precisely measure the complex dynamicity and the binding strength to assess the binding stability between a ligand and its target (Alamri et al. 2020; El Hassab et al. 2020). Moreover, MDS computations are vastly superior to other drug design techniques as they are based on millions of conformations. For this purpose, three MDS experiments were performed using free ATV, free zein, and the ATV-zein complex. The RMSD values for the three systems were determined to evaluate the binding stability between ATV and zein compared to their respective free forms. As anticipated, the RMSD values of the ATV–zein complex were significantly lower than those of the free forms. As illustrated in Fig. 3II, the average RMSD values reached 1.81, 8.75, and 4.57 Å for ATV–zein complex, free ATV, and free zein, respectively. This confirmed the efficient ability of zein protein to encapsulate ATV by creating a robust complex stabilized by numerous interactions.
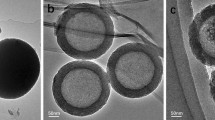
Morphological examination
As illustrated in Fig. 4, the TEM images demonstrated a well-defined spherical structure of the nanoparticles formed from a zein core that was covered entirely with a lecithin coat, which was consistent with the images formerly illustrated by Fu et al. (Fu et al. 2021). The particle size of the Opt-LCZN observed from the TEM images agreed with that previously evaluated using the Zetasizer.
Thermal analysis using differential scanning calorimetry (DSC) technique
As illustrated in Supplementary Fig. 1I, the characteristic peak of ATV was observed at 154 °C in the thermogram of the pure drug corresponding to the drug melting point and emphasizing its crystallinity. The peak of the drug was also well-preserved in the thermogram of the physical mixture, excluding any physical or chemical interactions between the drug and the other associated constituents. Conversely, the peak of the drug was absent in the thermogram of the Opt-LCZN, suggesting the development of amorphous ATV ascribed to the efficient encapsulation of the drug molecules into nanoparticles (Li et al. 2013).
Fourier-transform infrared (FTIR) spectroscopy
As illustrated in Supplementary Fig. 1II, the FTIR spectrum of ATV exhibited two sharp peaks at 3251 cm-1 and 3665 cm-1 representing the O–H groups. Meanwhile, the N–H stretching represented its peak at 3364 cm-1. However, the C=O amide group and the C–N stretching exhibited their peaks at 1634 cm-1 and 1316 cm-1, respectively. The aromatic out-of-plane bending showed its characteristic peak at 695 cm-1 (Abdelkader et al. 2021). Regarding the IR spectrum of the physical mixture, the drug bands appeared in decreased intensities, confirming the absence of any drug-excipient interference. On the contrary, the drug bands disappeared from the IR spectrum of the Opt-LCZN, which might have proved the efficient encapsulation of the drug into the nanoparticles (Khan et al. 2019).
Investigating the effect of aging
During storage, the Opt-LCZN ruled out any aggregation or physical changes upon standing. As shown in Table 3, after 30 and 90 days of storage, there was no significant difference in the mean values of EE%, PS, PDI, and ZP between the freshly prepared Opt-LCZN and the stored ones (p > 0.05). Furthermore, it was observed that both release curves obtained from the stored formulations were approximately symmetrical to that of the fresh one, exhibiting a similarity factor (ƒ2) of 78 after 30 days and 73 after 90 days. In light of our findings, it was observed that the Opt-LCZN was not affected by storage conditions. This might be ascribed to the aforementioned stabilizing effect provided by the lecithin surface coat and the PEGylation offered by Gelucire® 44/14 molecules causing further steric stabilization (Chuacharoen and Sabliov 2016; El-Lakany et al. 2018). In addition, the surface negative charge imparted by the lecithin coat maintains the electrostatic stabilization of the nanoparticles (Dai et al. 2017; Yuan et al. 2019).
Preparation of Opt-LCZN loaded thermogel (Opt-LCZN-thermogel)
The Opt-LCZN was loaded into a thermogel to obtain an easily injectable depot delivery system that forms a gel upon intra-articular administration, enhancing the drug's residence time in the knee joint.
Characterization of the Opt-LCZN-thermogel
Sol–gel transition temperature (Tsol-gel) measurement
The Opt-LCZN-thermogel formed a viscous gel with no signs of flowability at 35.25 ± 0.49 °C which is considered suitable for the intra-articular treatment of knee osteoarthritis (Zhang et al. 2021b).
Determination of gelation time
Gelation time is a critical parameter in thermogel formulations, where very rapid gelation in a couple of seconds may block the syringe needle resulting in incomplete dosage administration. Conversely, a prolonged gelation time may decrease the drug concentration at the target tissue due to the diffusion of the formulation to the surrounding tissues. At the predetermined Tsol-gel temperature, the Opt-LCZN-thermogel appeared to form a stiff gel within 57.5 ± 3.53 s which was suitable for its intra-articular administration (Venkatesh et al. 2020).
Viscosity evaluation and rheological study
The reversibility of the viscosity of the Opt-LCZN-thermogel upon changing the temperature was confirmed by determining the viscosity at ambient temperature (25 °C) and the physiological temperature (35 °C). The viscosity increased from 3135.5 cps at 25 °C to 13,335.5 cps at 35 °C; this fourfold increase in viscosity ensured the development of a viscous gel at physiological temperature.
As illustrated in Supplementary Fig. 2, the Opt-LCZN-thermogel at both temperatures (25 °C and 35 °C) demonstrated a pseudoplastic flow where the viscosity was inversely proportional to the shear rate. This might be ascribed to the co-existence of polymers such as poloxamer 407 and HA (Jones et al. 2003; Dong et al. 2013). This flow is considered ideal in parenteral formulations as low viscosity is required during application at ambient temperature, and high viscosity is preferable after its intra-articular administration for good drug retention at the osteoarthritic knee joint.
Syringeability
The Opt-LCZN-thermogel easily passed through the 21-gauge syringe needle and the syringe plunger required only effortless pressure to facilitate its intra-articular administration.
Assessment of the release rate of ATV from the Opt-LCZN-thermogel
As illustrated in Fig. 2 at 144 h, approximately 90% of ATV was released from Opt-LCZN, while approximately 70% of the drug was released from ATV-thermogel. On the other hand, 50% of the drug was released from Opt-LCZN-thermogel. The drug release from the latter was significantly prolonged (p = 0.0001) due to the hindrance effect caused by embedding the formulation within the gel matrix, which resulted in a more viscid diffusion pathway (Şenyiğit et al. 2015). The mathematical modeling of the drug release revealed that the Opt-LCZN followed the first-order model with the highest R2 (0.8095) indicating that the drug release was dose-dependent (Xiao et al. 2020; Gomaa et al. 2023). While the ATV-thermogel and Opt-LCZN-thermogel followed the Higuchi diffusion model with the highest R2 (0.6930 and 0.8342, respectively), and this result was parallel to that previously observed by Petit et al. (Petit et al. 2015). Additionally, the mechanism of drug release was determined by applying the Korsmeyer–Peppas model according to the value of the release exponent (n) where n ≤ 0.5 indicates a Fickian diffusion mechanism, while 0.45 < n < 0.89 indicates a non-Fickian or anomalous behavior of drug release (Vatankhah 2018). The n value of Opt-LCZN, ATV-thermogel, and Opt-LCZN-thermogel was 0.228, 0.282 and 0.318 (n ≤ 0.45) which depicted a Fickian diffusion mechanism (Dewan et al. 2015; Kang et al. 2021).
Terminal sterilization
Comparing the release profiles of the Opt-LCZN-thermogel before and after gamma sterilization, it was observed that the sterilization process did not affect its rate of drug release, as illustrated in Fig. 2. In addition, the computed f2 was found to be 74, which lies in the accepted range (51–100).
In-vivo study
Our study aims to alleviate arthritic inflammation by administering ATV intraarticularly. The CFA model was employed because of its significance to the physiological changes that occur with inflammatory arthritis.
Evaluation of knee joint swelling
As illustrated in Fig. 5, the percentage change in knee swelling during the experiment was computed at different time points (days 1, 7, 14, 21, and 28). A progressive significant elevation in the joint diameter after CFA induction was observed, with maximum swelling at day 14 in all the arthritic groups (p ≤ 0.001) as compared to the control group. On day 21, a significant decrease in knee swelling was observed in ATV-Opt-LCZN-thermogel group and ATV-thermogel group with values of 35.5 ± 5.47% and 43.17 ± 3.18%, respectively as compared to the CFA group (p ≤ 0.001). Meanwhile, on day 28, further reduction in knee swelling was detected in ATV-Opt-LCZN-thermogel group (24.67 ± 3.88%) and ATV-thermogel group (37.5 ± 3.56%) as compared to the CFA group (p ≤ 0.001). In accordance, a previous study conducted that the administration of ATV reduced knee joint swelling in osteoarthritic rats suggesting the ATV anti-hyperalgesic effect (Pathak et al. 2014).
Effect of different formulations of ATV on the mean percentage change in knee joint swelling at different time points, and the serum levels of the pro-inflammatory mediators (IL-6, IL-1β, and TNF-α) and the anti-inflammatory mediators (IL-4, IL-10, and IL-13). Values are means ± SD, (n = 6). ANOVA followed by Tukey’s post hoc test (p < 0.001) aSignificant from the control group, bSignificant from the CFA control group, cSignificant from the ATV-Opt-LCZN-thermogel group
Behavioral testing: knee bend test
The nociception caused by the joint movement was assessed using the knee bend test (Ferreira-Gomes et al. 2012). As illustrated in Table 4, the behavioral alterations in CFA group were time-dependent, with the dramatic rise in nociception reported on day 28 (9.4 ± 0.57) and remained higher than in the control group throughout the experiment. On day 21, a significant score reduction was observed in the ATV-Opt-LCZN- thermogel group and ATV-thermogel group with values of 3.85 ± 0.67 and 5.6 ± 0.58, respectively as compared to the CFA group (p ≤ 0.001). Meanwhile, on day 28, a further significant reduction in nociception was noticed in ATV-Opt-LCZN-thermogel group (1.58 ± 0.36) and ATV-thermogel group (3.6 ± 0.45) as compared to the CFA group (p ≤ 0.001). Interestingly, rats treated with ATV-Opt-LCZN-thermogel presented a significant decrease in nociception by 31% and 58%, on days 21 and 28, respectively as compared to group IV treated with ATV-thermogel. Our results came along with a previous study, that investigated the effect of ATV on nociception in carrageenan osteoarthritic model (Gaballah et al. 2022). This might be due to the anti-inflammatory and immunomodulatory actions of ATV together with the modulation of pain and inflammation caused by HA (Larry W. Moreland 2003; Parsamanesh et al. 2019).
Assessment of pro-inflammatory mediators in serum
Inflammatory mediators such as cytokines and chemokines generated in joint tissues cause cartilage deterioration (Molnar et al. 2021). Pro-inflammatory cytokines including IL-6, IL-1ß and TNF-α impair homeostasis in the articular joints and cartilage matrix by stimulating chondrocytes to create additional inflammatory cytokines, amplifying an existing detrimental cellular response (Ansari et al. 2020). As illustrated in Fig. 5, when compared to the control group, it was observed that the CFA group showed a significant elevation in IL-1β (200.01 ± 7.34 pg/ml), IL-6 (99.98 ± 6.51 pg/ml), and TNF-α (122.4 ± 4.09 pg/ml) levels (p < 0.0001). However, when compared to the CFA group, rats treated with ATV-Opt-LCZN-thermogel showed a significant amelioration of IL-1β (70.75 ± 5.28 pg/ml), IL-6 (28.48 ± 2.82 pg/ml), and TNF-α (58.4 ± 3.54 pg/ml) levels, also rats treated with ATV-thermogel showed significant alleviation of IL-1β (92.48 ± 8.14 pg/ml), IL-6 (49.97 ± 4.74 pg/ml) and TNF-α (73.68 ± 3.11 pg/ml) levels (p < 0.0001). This noticeable down-regulation might be due to the anti-inflammatory effect of ATV where these results were parallel to that previously observed by Gaballah et al. (Gaballah et al. 2022). Moreover, such findings revealed that the ATV-Opt-LCZN-thermogel group showed a significant reduction of the aforementioned pro-inflammatory mediators as compared to the ATV-thermogel group. This might be due to the prolonged retention period of the drug that was encapsulated into the zein nanoparticles resulting in a depot action (Kim et al. 2015).
Assessment of anti-inflammatory mediators in serum
The resolution of inflammation in arthritis occurs as a consequence of the activation of type two T helper cells, which produce IL-4, IL-10, and IL-13 (Askari et al. 2019). As illustrated in Fig. 5, a significant reduction in IL-4, IL-10, and IL-13 levels to a value of 37.87 ± 3.3 pg/ml, 64.90 ± 6.7 pg/ml, and 75 ± 5.6 pg/ml respectively, was noticed in CFA group (p < 0.0001) when compared to the control group. However, a significant elevation of IL-4, IL-10, and IL-13 levels was observed in the ATV-Opt-LCZN-thermogel group and ATV-thermogel group. The level of anti-inflammatory mediators (IL-4, IL-10, and IL-13) was ameliorated in the ATV-Opt-LCZN-thermogel group with values of 107.8 ± 4.49 pg/ml, 145.9 ± 4.19 pg/ml, and 133.2 ± 4.75 pg/ml, respectively as compared to the CFA group (p < 0.0001). Meanwhile, the ATV-thermogel group showed a significant elevation of IL-4, IL-10, and IL-13 levels with values of 59.22 ± 4.6 pg/ml, 126.5 ± 9.7 pg/ml, and 105.3 ± 5.5 pg/ml, respectively. This up-regulation in both groups resulted from the concomitant administration of both ATV and HA (Yu et al. 2018). It is worth mentioning that the ATV-Opt-LCZN-thermogel group showed a significant increase in IL-4, IL-10, and IL-13 levels by 55%, 26%, and 15%, respectively as compared to the ATV-thermogel group. This enhancement that was noticeable in the ATV-Opt-LCZN-thermogel group may be attributed to the increase in the retention of ATV in the articular joints as a result of the biodegradable nature of zein nanoparticles which allowed for the sustained release of the drug (Morgen et al. 2013). Moreover, this significant alleviation in the levels of the inflammatory mediators observed in the ATV-Opt-LCZN-thermogel group explains its superiority in ameliorating knee swelling and nociception as compared to the ATV-thermogel group.
Histopathological examination
The histopathological examination of knee joints showed normal articular cartilage appearance with normal cellularity in the control group (Fig. 6a, b). Whereas in the CFA group, CFA intoxication prompted prominent histopathological changes in the form of a decrease in the thickness of the articular cartilage with extensive regions of erosion as compared to the control group (Fig. 6c). Although the surface of articular cartilage appeared to be smooth and uniform, the connection between articular cartilage and subchondral bone was flattened, creating vast bone marrow gaps. Moreover, the knee joint showed a narrow joint cavity, large destructing pannus composed of inflammatory cells and excess fibroblasts on articular cartilage and on menisci, ulcerated and hyperplastic synovial membrane with the villous formation, and marked sub-synovial inflammatory infiltrate (Fig. 6d). On the other hand, the ATV-Opt-LCZN-thermogel group exerted more obvious improvement effects as shown by minimal articular cartilage degeneration and mild sub-synovial inflammatory infiltrate as compared to the CFA group (Fig. 6e, f). Meanwhile, the ATV-thermogel group still exhibited huge areas of degenerative changes of chondrocytes and surrounding matrix accompanied by the hyperplastic synovial membrane and marked sub-synovial inflammatory infiltrate (Fig. 6g, h). The histopathological examination of the rat knee joints mirrored the outcomes of the previous tests where the decrease of the knee joint swelling and other pro-inflammatory mediators was associated with a considerable improvement in the histopathological manifestations of ATV-Opt-LCZN-thermogel group. These findings indicated the chondroprotective effect of ATV-Opt-LCZN-thermogel which might be attributed to the encapsulation of ATV into the zein nanoparticles.
Histopathological examination of knee joint edema in CFA-induced arthritis. a (Control group): joint showing average joint cavity (black arrow) with an intact synovial membrane (blue arrow), average sub-synovial tissue (yellow arrow), average articular cartilage with intact superficial layer (red arrow), and average menisci (green arrow) (H&E X 100). b (Control group): another view showing the average joint cavity (black arrow) with an intact synovial membrane (blue arrow), average sub-synovial blood vessels (yellow arrow), average articular cartilage with intact superficial layer (red arrow), and average menisci (green arrow) (H&E X 200). c (CFA group): joint showing narrow joint cavity (black arrow), large destructing Pannus on articular cartilage (blue arrow) and on menisci (red arrow), and marked sub-synovial inflammatory infiltrate (yellow arrow) (H&E X 100). d (CFA group): high power view showing large destructing Pannus on articular cartilage (black arrow), ulcerated synovial membrane (red arrow) with marked sub-synovial inflammatory infiltrate (yellow arrow) (H&E X 200). e (ATV-Opt-LCZN-thermogel group): joint showing narrow joint cavity (black arrow), small non-destructing Pannus on articular cartilage (blue arrow) and on menisci (red arrow) (H&E X 100). f (ATV-Opt-LCZN-thermogel group): high power view showing narrow joint cavity (black arrow), small non-destructing Pannus on articular cartilage (blue arrow) and on menisci (red arrow), and mild sub-synovial inflammatory infiltrate (yellow arrow) (H&E X 200). g (ATV-thermogel group): joint showing average joint cavity (black arrow), small non-destructing Pannus on articular cartilage (blue arrow) and on menisci (red arrow) (H&E X 100). h (ATV-thermogel group): high power view showing small non-destructing Pannus with fibrin deposits on menisci (black arrow) and intact synovial membrane (red arrow) with marked sub-synovial inflammatory infiltrate (blue arrow) (H&E X 200)
Conclusion
The present work established an injectable intra-articular depot delivery system of atorvastatin calcium (ATV) for ameliorating osteoarthritis (OA). Statistical optimization of ATV loaded into lecithin-coated zein nanoparticles succeeded in generating an optimized formulation (Opt-LCZN), which was further loaded into a thermogel (Opt-LCZN-thermogel) to be easily administered and to resist rapid clearance at the knee joint. The in-silico studies confirmed the efficient ability of zein to encapsulate the drug. The in-vivo studies highlighted the powerful anti-inflammatory properties of ATV as a key mechanism underlying the promising chondroprotective effect against the OA-induced model. In light of our outcomes, it is observed that the intra-articular administration of ATV loaded onto Opt-LCZN-thermogel is a well-advocated novel delivery system that can be an appropriate alternative therapy for OA.
Data availability
All data will be made available upon request.
References
Abd Ellah NH, Abdel-Aleem JA, Abdo MN et al (2019) Efficacy of ketoconazole gel-flakes in treatment of vaginal candidiasis: formulation, in vitro and clinical evaluation. Int J Pharm 567:118472. https://doi.org/10.1016/j.ijpharm.2019.118472
Abdelkader DH, Abosalha AK, Khattab MA et al (2021) A novel sustained anti-inflammatory effect of atorvastatin—calcium PLGA nanoparticles: in vitro optimization and in vivo evaluation. Pharmaceutics 13(10):1658. https://doi.org/10.3390/PHARMACEUTICS13101658
Abdellatif MM, Khalil IA, Elakkad YE et al (2020) Formulation and characterization of sertaconazole nitrate mucoadhesive liposomes for vaginal candidiasis. Int J Nanomed 15:4079–4090. https://doi.org/10.2147/IJN.S250960
Aboumanei MH, Mahmoud AF (2020) Design and development of a proniosomal transdermal drug delivery system of caffeine for management of migraine: In vitro characterization, 131I-radiolabeling and in vivo biodistribution studies. Process Biochem 97:201–212. https://doi.org/10.1016/j.procbio.2020.07.018
Ahmed OAA (2018) Development and single dose clinical pharmacokinetics investigation of novel zein assisted- alpha lipoic acid nanoencapsulation of vardenafil. Sci Rep 8(1):15802. https://doi.org/10.1038/s41598-018-34235-8
Alamri MA, Tahir UQM, Mirza MU et al (2020) Pharmacoinformatics and molecular dynamics simulation studies reveal potential covalent and FDA-approved inhibitors of SARS-CoV-2 main protease 3CLpro. J Biomol Struct Dyn 39(13):4936–4948. https://doi.org/10.1080/07391102.2020.1782768
Alazragi RS, Baeissa HM (2023) Chondroprotective effects of grapefruit (Citrus paradisi Macfad) juice in a complete Freund’s adjuvant rat model of knee osteoarthritis. Nutrients 15(4):798. https://doi.org/10.3390/NU15040798
Albash R, Ragaie MH, Hassab MAE et al (2022) Fenticonazole nitrate loaded trans-novasomes for effective management of tinea corporis: design characterization, in silico study, and exploratory clinical appraisal. Drug Deliv 29:1100–1111. https://doi.org/10.1080/10717544.2022.2057619
Ansari MY, Ahmad N, Haqqi TM (2020) Oxidative stress and inflammation in osteoarthritis pathogenesis: role of polyphenols. Biomed Pharmacother 129:110452. https://doi.org/10.1016/j.biopha.2020.110452
Askari VR, Baradaran Rahimi V, Tabatabaee SA, Shafiee-Nick R (2019) Combination of Imipramine, a sphingomyelinase inhibitor, and β-caryophyllene improve their therapeutic effects on experimental autoimmune encephalomyelitis (EAE). Int Immunopharmacol 77:105923. https://doi.org/10.1016/J.INTIMP.2019.105923
Barsante MM, Roffê E, Yokoro CM et al (2005) Anti-inflammatory and analgesic effects of atorvastatin in a rat model of adjuvant-induced arthritis. Eur J Pharmacol 516:282–289. https://doi.org/10.1016/j.ejphar.2005.05.005
Bhattacharjee J, Jogdand S, Goswami S et al (2017) Evaluation of analgesic activity of simvastatin and atorvastatin in Wistar rats: an experimental study. Natl J Physiol Pharm Pharmacol 7:1031–1035. https://doi.org/10.5455/njppp.2017.7.0411710052017
Bindu S, Mazumder S, Bandyopadhyay U (2020) Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: a current perspective. Biochem Pharmacol 180:114147. https://doi.org/10.1016/J.BCP.2020.114147
Case DA, Cheatham TE, Darden T et al (2005) The Amber biomolecular simulation programs. J Comput Chem 26:1668–1688. https://doi.org/10.1002/JCC.20290
Chang MC, Chiang PF, Kuo YJ et al (2021) Hyaluronan-loaded liposomal dexamethasone-diclofenac nanoparticles for local osteoarthritis treatment. Int J Mol Sci 22(2):665. https://doi.org/10.3390/IJMS22020665
Choudhary A, Rana AC, Aggarwal G et al (2012) Development and characterization of an atorvastatin solid dispersion formulation using skimmed milk for improved oral bioavailability. Acta Pharm Sin B 2:421–428. https://doi.org/10.1016/j.apsb.2012.05.002
Chuacharoen T, Sabliov CM (2016) Stability and controlled release of lutein loaded in zein nanoparticles with and without lecithin and pluronic F127 surfactants. Colloids Surf A Physicochem Eng Asp 503:11–18. https://doi.org/10.1016/j.colsurfa.2016.04.038
Dai L, Sun C, Wang D, Gao Y (2016) The interaction between zein and lecithin in ethanol-Water solution and characterization of zein-Lecithin composite colloidal nanoparticles. PLoS ONE 11(11):e0167172. https://doi.org/10.1371/journal.pone.0167172
Dai L, Sun C, Li R et al (2017) Structural characterization, formation mechanism and stability of curcumin in zein-lecithin composite nanoparticles fabricated by antisolvent co-precipitation. Food Chem 237:1163–1171. https://doi.org/10.1016/j.foodchem.2017.05.134
Dewan M, Bhowmick B, Sarkar G et al (2015) Effect of methyl cellulose on gelation behavior and drug release from poloxamer based ophthalmic formulations. Int J Biol Macromol 72:706–710. https://doi.org/10.1016/J.IJBIOMAC.2014.09.021
Dinc M, Bilgen MS, Kucukalp A, Bilgen OF (2012) An assessment of the chondroprotective effects of intra-articular application of statin and tetracycline on early-stage experimental osteoarthritis. ISRN Orthop 2012:182097. https://doi.org/10.5402/2012/182097
Dong J, Jiang D, Wang Z et al (2013) Intra-articular delivery of liposomal celecoxib-hyaluronate combination for the treatment of osteoarthritis in rabbit model. Int J Pharm 441:285–290. https://doi.org/10.1016/j.ijpharm.2012.11.031
Dong F, Dong X, Zhou L et al (2016) Doxorubicin-loaded biodegradable self-assembly zein nanoparticle and its anti-cancer effect: preparation, in vitro evaluation, and cellular uptake. Colloids Surf B Biointerfaces 140:324–331. https://doi.org/10.1016/j.colsurfb.2015.12.048
Dwajani S, Harish Kumar V, Keerthi D (2012) Atorvastatin and simvastatin as analgesic agents in experimental models. J Basic Clin Pharm 3:332. https://doi.org/10.4103/0976-0105.105334
El Assasy AEHI, Younes NF, Makhlouf AIA (2019) Enhanced oral absorption of amisulpride via a nanostructured lipid carrier-based capsules: development, optimization applying the desirability function approach and in vivo pharmacokinetic study. AAPS PharmSciTech 20(2):82. https://doi.org/10.1208/s12249-018-1283-x
El Hassab MA, Shoun AA, Al-Rashood ST et al (2020) Identification of a new potential SARS-COV-2 RNA-dependent RNA polymerase inhibitor via combining fragment-based drug design, docking, molecular dynamics, and MM-PBSA calculations. Front Chem 8:915. https://doi.org/10.3389/FCHEM.2020.584894/BIBTEX
Elgendy HA, Makky AMA, Elakkad YE et al (2023) Syringeable atorvastatin loaded eugenol enriched PEGylated cubosomes in-situ gel for the intra-pocket treatment of periodontitis: statistical optimization and clinical assessment. Drug Deliv 30(1):2162159. https://doi.org/10.1080/10717544.2022.2162159
El-Gogary RI, Rubio N, Wang JTW et al (2014) Polyethylene glycol conjugated polymeric nanocapsules for targeted delivery of quercetin to folate-expressing cancer cells in vitro and in vivo. ACS Nano 8:1384–1401. https://doi.org/10.1021/nn405155b
El-Hasab MAEM, El-Bastawissy EE, El-Moselhy TF (2017) Identification of potential inhibitors for HCV NS3 genotype 4a by combining protein–ligand interaction fingerprint, 3D pharmacophore, docking, and dynamic simulation. J Biomol Struct Dyn 36:1713–1727. https://doi.org/10.1080/07391102.2017.1332689
El-Hassab MAEM, El-Bastawissy EE, El-Moselhy TF (2020) Identification of potential inhibitors for HCV NS5b of genotype 4a by combining dynamic simulation, protein–ligand interaction fingerprint, 3D pharmacophore, docking and 3D QSAR. J Biomol Struct Dyn 38:4521–4535. https://doi.org/10.1080/07391102.2019.1685005/SUPPL_FILE/TBSD_A_1685005_SM0645.DOCX
El-Helaly SN, Elbary AA, Kassem MA, El-Nabarawi MA (2017) Electrosteric stealth rivastigmine loaded liposomes for brain targeting: preparation, characterization, ex vivo, bio-distribution and in vivo pharmacokinetic studies. Drug Deliv 24:692–700. https://doi.org/10.1080/10717544.2017.1309476
El-Lakany SA, Elgindy NA, Helmy MW et al (2018) Lactoferrin-decorated vs PEGylated zein nanospheres for combined aromatase inhibitor and herbal therapy of breast cancer. Expert Opin Drug Deliv 15:835–850. https://doi.org/10.1080/17425247.2018.1505858
El-Leithy ES, Makky AM, Khattab AM, Hussein DG (2018) Optimization of nutraceutical coenzyme Q10 nanoemulsion with improved skin permeability and anti-wrinkle efficiency. Drug Dev Ind Pharm 44:316–328. https://doi.org/10.1080/03639045.2017.1391836
El-Nabarawi MA, Shamma RN, Farouk F, Nasralla SM (2018) Dapsone-loaded invasomes as a potential treatment of acne: preparation, characterization, and in vivo skin deposition assay. AAPS PharmSciTech 19:2174–2184. https://doi.org/10.1208/S12249-018-1025-0/TABLES/4
Elsewedy HS, Dhubiab BEA, Mahdy MA, Elnahas HM (2020) Development, optimization, and evaluation of PEGylated brucine-loaded PLGA nanoparticles. Drug Deliv 27:1134–1146. https://doi.org/10.1080/10717544.2020.1797237
El-Tedawy DM, Abd-Alhaseeb MM, Helmy MW, Ghoneim AI (2020) Systemic bee venom exerts anti-arthritic and anti-inflammatory properties in a rat model of arthritis. Biomed Rep 13(4):20. https://doi.org/10.3892/br.2020.1327
Ferreira-Gomes J, Adães S, Mendonça M, Castro-Lopes JM (2012) Analgesic effects of lidocaine, morphine and diclofenac on movement-induced nociception, as assessed by the Knee-Bend and CatWalk tests in a rat model of osteoarthritis. Pharmacol Biochem Behav 101:617–624. https://doi.org/10.1016/j.pbb.2012.03.003
Fouda NH, Abdelrehim RT, Hegazy DA, Habib BA (2018) Sustained ocular delivery of dorzolamide-HCL via proniosomal gel formulation: In-vitro characterization, statistical optimization, and in-vivo pharmacodynamic evaluation in rabbits. Drug Deliv 25:1340–1349. https://doi.org/10.1080/10717544.2018.1477861
Fu W, Liang Y, Xie Z et al (2021) Preparation and evaluation of lecithin/zein hybrid nanoparticles for the oral delivery of Panax notoginseng saponins. Eur J Pharm Sci 164:105882. https://doi.org/10.1016/j.ejps.2021.105882
Gaballah A, Genedy D, Ghayaty E et al (2022) Standardized study of atorvastatin possible osteoarthritis disease-modifying effect in a rat model of osteoarthritis. Fundam Clin Pharmacol 36:296–305. https://doi.org/10.1111/fcp.12730
Gagliardi A, Voci S, Giuliano E et al (2021) Phospholipid/zein hybrid nanoparticles as promising carriers for the protection and delivery of all-trans retinoic acid. Mater Sci Eng C Mater Biol Appl 128:112331. https://doi.org/10.1016/j.msec.2021.112331
Gomaa E, Eissa NG, Ibrahim TM et al (2023) Development of depot PLGA-based in-situ implant of Linagliptin: sustained release and glycemic control. Saudi Pharm J 31:499–509. https://doi.org/10.1016/J.JSPS.2023.02.002
Hamdi M, Abdel-Bar HM, Elmowafy E et al (2020) An integrated vitamin E-coated polymer hybrid nanoplatform: a lucrative option for an enhanced in vitro macrophage retention for an anti-hepatitis B therapeutic prospect. PLoS ONE 15(1):e0227231. https://doi.org/10.1371/journal.pone.0227231
Hanafy AS, El-Ganainy SO (2020) Thermoresponsive Hyalomer intra-articular hydrogels improve monoiodoacetate-induced osteoarthritis in rats. Int J Pharm 573:118859. https://doi.org/10.1016/j.ijpharm.2019.118859
Hathout RM, Woodman TJ (2012) Applications of NMR in the characterization of pharmaceutical microemulsions. J Control Release 161(1):62–72. https://doi.org/10.1016/j.jconrel.2012.04.032
Hu D, Lin C, Liu L et al (2012) Preparation, characterization, and in vitro release investigation of lutein/zein nanoparticles via solution enhanced dispersion by supercritical fluids. J Food Eng 109:545–552. https://doi.org/10.1016/j.jfoodeng.2011.10.025
Jansen MP, Mastbergen SC (2022) Joint distraction for osteoarthritis: clinical evidence and molecular mechanisms. Nat Rev Rheumatol 18(1):35–46. https://doi.org/10.1038/s41584-021-00695-y
Jones DS, Lawlor MS, Woolfson AD (2003) Rheological and mucoadhesive characterization of polymeric systems composed of poly(methylvinylether-co-maleic anhydride) and poly(vinylpyrrolidone), designed as platforms for topical drug delivery. J Pharm Sci 92(5):995–1007. https://doi.org/10.1002/jps.10357
Jones IA, Togashi R, Wilson ML et al (2019) Intra-articular treatment options for knee osteoarthritis. Nat Rev Rheumatol 15(2):77–90. https://doi.org/10.1038/s41584-018-0123-4
Jung YS, Park W, Park H et al (2017) Thermo-sensitive injectable hydrogel based on the physical mixing of hyaluronic acid and Pluronic F-127 for sustained NSAID delivery. Carbohydr Polym 156:403–408. https://doi.org/10.1016/j.carbpol.2016.08.068
Kamel R, Salama AH, Mahmoud AA (2016) Development and optimization of self-assembling nanosystem for intra-articular delivery of indomethacin. Int J Pharm 515:657–668. https://doi.org/10.1016/j.ijpharm.2016.10.063
Kang S, He Y, Yu DG et al (2021) Drug–zein@lipid hybrid nanoparticles: electrospraying preparation and drug extended release application. Colloids Surf B Biointerfaces 201:111629. https://doi.org/10.1016/J.COLSURFB.2021.111629
Kawakami K, Miyoshi K, Ida Y (2004) Solubilization behavior of poorly soluble drugs with combined use of gelucire 44/14 and cosolvent. J Pharm Sci 93(6):1471–1479. https://doi.org/10.1002/jps.20067
Khan MA, Yue C, Fang Z et al (2019) Alginate/chitosan-coated zein nanoparticles for the delivery of resveratrol. J Food Eng 258:45–53. https://doi.org/10.1016/j.jfoodeng.2019.04.010
Khan MA, Chen L, Liang L (2021) Improvement in storage stability and resveratrol retention by fabrication of hollow zein-chitosan composite particles. Food Hydrocoll 113:106447. https://doi.org/10.1016/j.foodhyd.2020.106477
Kim SR, Ho MJ, Lee E et al (2015) Cationic PLGA/eudragit RL nanoparticles for increasing retention time in synovial cavity after intra-articular injection in knee joint. Int J Nanomed 10:5263–5271. https://doi.org/10.2147/IJN.S88363
Krtalić I, Radošević S, Hafner A et al (2018) D-Optimal design in the development of rheologically improved in situ forming ophthalmic gel. J Pharm Sci 107:1562–1571. https://doi.org/10.1016/j.xphs.2018.01.019
Li KK, Yin SW, Yin YC et al (2013) Preparation of water-soluble antimicrobial zein nanoparticles by a modified antisolvent approach and their characterization. J Food Eng 119:343–352. https://doi.org/10.1016/j.jfoodeng.2013.05.038
Liu-Bryan R, Terkeltaub R (2015) Emerging regulators of the inflammatory process in osteoarthritis. Nat Rev Rheumatol 11(1):35–44. https://doi.org/10.1038/nrrheum.2014.162
Mayol L, Quaglia F, Borzacchiello A et al (2008) A novel poloxamers/hyaluronic acid in situ forming hydrogel for drug delivery: Rheological, mucoadhesive and in vitro release properties. Eur J Pharm Biopharm 70:199–206. https://doi.org/10.1016/j.ejpb.2008.04.025
Mohamed HI, El-Kamel AH, Hammad GO et al (2022) Design of targeted flurbiprofen biomimetic nanoparticles for management of arthritis: in vitro and in vivo appraisal. Pharmaceutics 14(1):140. https://doi.org/10.3390/PHARMACEUTICS14010140
Mok SW, Fu SC, Cheuk YC et al (2020) Intra-articular delivery of quercetin using thermosensitive hydrogel attenuate cartilage degradation in an osteoarthritis rat model. Cartilage 11:490–499. https://doi.org/10.1177/1947603518796550
Molnar V, Matišić V, Kodvanj I et al (2021) Cytokines and chemokines involved in osteoarthritis pathogenesis. Int J Mol Sci 22(17):9208. https://doi.org/10.3390/ijms22179208
Moreland LW (2003) Intra-articular hyaluronan (hyaluronic acid) and hylans for the treatment of osteoarthritis: mechanisms of action. Arthritis Res Ther 5:1–4. https://doi.org/10.1186/ar623
Morgen M, Tung D, Boras B et al (2013) Nanoparticles for improved local retention after intra-articular injection into the knee joint. Pharm Res 30:257–268. https://doi.org/10.1007/S11095-012-0870-X/FIGURES/9
Morsi N, Ghorab D, Refai H, Teba H (2016) Ketoroloac tromethamine loaded nanodispersion incorporated into thermosensitive in situ gel for prolonged ocular delivery. Int J Pharm 506:57–67. https://doi.org/10.1016/j.ijpharm.2016.04.021
Mossiat C, Laroche D, Prati C et al (2015) Association between arthritis score at the onset of the disease and long-term locomotor outcome in adjuvant-induced arthritis in rats. Arthritis Res Ther 17:1–12. https://doi.org/10.1186/S13075-015-0700-8/FIGURES/6
National Research Council (2011) Guide for the care and use of laboratory animals: eighth edition. National Research Council, Division on Earth and Life Studies, Institute for Laboratory Animal Research, Committee for the Update of the Guide for the Care and Use of Laboratory Animals, Google Books. https://books.google.com.eg/books?hl=en&lr=&id=Vp5mgXtxYdQC&oi=fnd&pg=PP2&dq=Care+CftUotGft,+and+Use+of+Laboratory+Animals+IfLAR.+Guide+for+the+care+and+use+of+laboratory+animals.+Washington:+The+National+Academies+Press%3B+2011&ots=Fs_c65KHm7&sig=twjv9L3kezfdrigTxUcIliOT27c&redir_esc=y#v=onepage&q&f=false
Parsamanesh N, Moossavi M, Bahrami A et al (2019) NLRP3 inflammasome as a treatment target in atherosclerosis: a focus on statin therapy. Int Immunopharmacol 73:146–155. https://doi.org/10.1016/J.INTIMP.2019.05.006
Pascoli M, de Lima R, Fraceto LF (2018) Zein nanoparticles and strategies to improve colloidal stability: a mini-review. Front Chem 6:6. https://doi.org/10.3389/fchem.2018.00006
Pathak NN, Balaganur V, Lingaraju MC et al (2014) Effect of atorvastatin, a HMG-CoA reductase inhibitor in monosodium iodoacetate-induced osteoarthritic pain: implication for osteoarthritis therapy. Pharmacol Rep 67(3):513–519. https://doi.org/10.1016/J.PHAREP.2014.12.005
Pathak NN, Lingaraju MC, Balaganur V et al (2015) Anti-inflammatory and chondroprotective effects of atorvastatin in a cartilage explant model of osteoarthritis. Inflamm Res 64(3–4):161–169. https://doi.org/10.1007/s00011-014-0794-5
Petit A, Redout EM, van de Lest CH et al (2015) Sustained intra-articular release of celecoxib from in situ forming gels made of acetyl-capped PCLA-PEG-PCLA triblock copolymers in horses. Biomaterials 53:426–436. https://doi.org/10.1016/J.BIOMATERIALS.2015.02.109
Phillips JC, Braun R, Wang W et al (2005) Scalable molecular dynamics with NAMD. J Comput Chem 26:1781–1802. https://doi.org/10.1002/JCC.20289
Radwan SAA, El-Maadawy WH, Yousry C et al (2020) Zein/phospholipid composite nanoparticles for successful delivery of gallic acid into aHSCs: influence of size, surface charge, and vitamin A coupling. Int J Nanomed 15:7995–8018. https://doi.org/10.2147/IJN.S270242
Robinson WH, Lepus CM, Wang Q et al (2016) Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat Rev Rheumatol 12(10):580–592. https://doi.org/10.1038/nrrheum.2016.136
Ronaldson KJ, O’shea JM, Boyd IW (2006) Risk factors for rhabdomyolysis with simvastatin and atorvastatin. Drug Saf 29(11):1061–1067. https://doi.org/10.2165/00002018-200629110-00005
Salama AH, Abousamra MM et al (2020) Promising bioadhesive ofloxacin-loaded polymeric nanoparticles for the treatment of ocular inflammation: formulation and in vivo evaluation. Drug Deliv Transl Res 11(5):1943–1957. https://doi.org/10.1007/s13346-020-00856-8/Published
Şenyiğit ZA, Karavana SY, İlem-Özdemir DI et al (2015) Design and evaluation of an intravesical delivery system for superficial bladder cancer: preparation of gemcitabine HCl-loaded chitosan-thioglycolic acid nanoparticles and comparison of chitosan/poloxamer gels as carriers. Int J Nanomed 10:6493–6507. https://doi.org/10.2147/IJN.S93750
Shukla R, Cheryan M (2001) Zein: the industrial protein from corn. Ind Crops Prod 13(3):171–192. https://doi.org/10.1016/S0926-6690(00)00064-9
Sokol MB, Chirkina MV, Yabbarov NG et al (2022) Structural optimization of platinum drugs to improve the drug-loading and antitumor efficacy of PLGA nanoparticles. Pharmaceutics 14:2333. https://doi.org/10.3390/PHARMACEUTICS14112333/S1
Souzandeh H, Netravali AN (2019) Self-healing of ‘green’ thermoset zein resins with irregular shaped waxy maize starch-based/poly(D, L-lactic-co-glycolic acid) microcapsules. Compos Sci Technol 183:107831. https://doi.org/10.1016/j.compscitech.2019.107831
Vatankhah E (2018) Rosmarinic acid-loaded electrospun nanofibers: in vitro release kinetic study and bioactivity assessment. Eng Life Sci 18:732–742. https://doi.org/10.1002/ELSC.201800046
Venkatesh MP, Kumar TP, Pai DR (2020) Targeted drug delivery of methotrexate in situ gels for the treatment of rheumatoid arthritis. Saudi Pharm J 28:1548–1557. https://doi.org/10.1016/J.JSPS.2020.10.003
Vilar S, Cozza G, Moro S (2008) Medicinal chemistry and the molecular operating environment (MOE): application of QSAR and molecular docking to drug discovery. Curr Top Med Chem 8:1555–1572. https://doi.org/10.2174/156802608786786624
Watterson JR, Esdaile M (2000) Viscosupplementation therapeutic mechanisms and clinical potential in osteoarthritis of the knee. Perspect Modern Orthop JAAOS 8(5):277–284
Xiao P, Qi P, Chen J et al (2020) The effect of polymer blends on initial release regulation and in vitro-in vivo relationship of peptides loaded PLGA-hydrogel microspheres. Int J Pharm 591:119964. https://doi.org/10.1016/J.IJPHARM.2020.119964
Younes NF, Habib BA (2022) Augmented local skin accumulation efficiency of sertaconazole nitrate via glycerosomal hydrogel: formulation, statistical optimization, ex vivo performance and in vivo penetration. J Drug Deliv Sci Technol 72:103364. https://doi.org/10.1016/j.jddst.2022.103364
Younes NF, Abdel-Halim SA, Elassasy AI (2018) Corneal targeted sertaconazole nitrate loaded cubosomes: preparation, statistical optimization, in vitro characterization, ex vivo permeation and in vivo studies. Int J Pharm 553:386–397. https://doi.org/10.1016/j.ijpharm.2018.10.057
Yu W, Xu P, Huang G, Liu L (2018) Clinical therapy of hyaluronic acid combined with platelet-rich plasma for the treatment of knee osteoarthritis. Exp Ther Med 16:2119–2125. https://doi.org/10.3892/etm.2018.6412
Yuan Y, Li H, Liu C et al (2019) Fabrication and characterization of lutein-loaded nanoparticles based on zein and sophorolipid: enhancement of water solubility, stability, and bioaccessibility. J Agric Food Chem 67(43):11977–11985. https://doi.org/10.1021/acs.jafc.9b05175
Zhang L, Shen W, Luan J et al (2015) Sustained intravitreal delivery of dexamethasone using an injectable and biodegradable thermogel. Acta Biomater 23:271–281. https://doi.org/10.1016/j.actbio.2015.05.005
Zhang H, van Os WL, Tian X et al (2021a) Development of curcumin-loaded zein nanoparticles for transport across the blood-brain barrier and inhibition of glioblastoma cell growth. Biomater Sci 9:7092–7103. https://doi.org/10.1039/d0bm01536a
Zhang T, Chen S, Dou H et al (2021b) Novel glucosamine-loaded thermosensitive hydrogels based on poloxamers for osteoarthritis therapy by intra-articular injection. Mater Sci Eng C 118:111352. https://doi.org/10.1016/j.msec.2020.111352
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by all authors.
Corresponding authors
Ethics declarations
Conflict of interest
All authors (H.A. Elgendy, A.M.A. Makky, Y.E. Elakkad, H.H. Awad, M.A.E. Hassab, and N.F. Younes) declare that they have no conflict of interest.
Informed consent
This study does not involve human subjects.
Consent for publication
This study does not contain any individual person’s data in any form.
Research involving animal participants
The animal studies were performed after receiving approval of the Research Ethical Committee at the Faculty of Pharmacy, Cairo University (PI (2947)).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elgendy, H.A., Makky, A.M.A., Elakkad, Y.E. et al. Atorvastatin loaded lecithin-coated zein nanoparticles based thermogel for the intra-articular management of osteoarthritis: in-silico, in-vitro, and in-vivo studies. J. Pharm. Investig. 54, 497–518 (2024). https://doi.org/10.1007/s40005-024-00666-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40005-024-00666-x