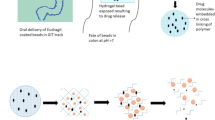
Abstract
Natural polysaccharides are widely used for development of colon-specific drug delivery systems. The present study was carried out to develop multi-particulate calcium pectinate (Ca-pectinate) formulations for colon-targeted delivery of lornoxicam. The formulations were developed using a combination of polycarbophil and low-methoxy amidated pectin. The beads were prepared using an ionotropic gelation technique. The effects of the polycarbophil and low-methoxy amidated pectin concentrations on the beads characteristics, encapsulation efficiency, swelling study and drug release performance were investigated. The optimized formulation was evaluated for its morphological characteristics. The in vitro drug release of the optimized formulation over a period of 12 h was 96.78 ± 1.35 %. The concentration of the polycarbophil was a decisive factor in sustaining drug release in the colon. The study revealed that optimized Ca-pectinate beads prepared with polycarbophil can efficiently encapsulate lornoxicam. It also showed that these beads can potentially be used for colon-specific delivery of lornoxicam.




Similar content being viewed by others
References
Anal AK, Stevens WF (2005) Chitosan-alginate multilayer beads for controlled release of ampicillin. Int J Pharm 290:45–54
Atyabi F, Vahabzadeh R, Dinarvand R (2004) Preparation of ethylcellulose coated gelatin microspheres as a multiparticulate colonic delivery system for 5-aminosalicylic acid. Iran J Pharm Res 3:81–86
Atyabi F, Majzoob S, Iman M, Salehi M, Dorkoosh F (2005) In vitro evaluation and modification of pectinate gel beads containing trimethyl chitosan, as a multi-particulate system for delivery of water-soluble macromolecules to colon. Carbohydr Polym 61:39–51
Avadi MR, Ghassemi AH, Sadeghi AM, Erfan M, Akbarzadeh A, Moghimi HR, Rafiee-Tehrani M (2004) Preparation and characterization of theophylline-chitosan beads as an approach to colon delivery. Iranian J Pharm Res 3:73–80
Chambin O, Dupuis G, Champion D, Voilley A, Pourcelot Y (2006) Colon-specific drug delivery: influence of solution reticulation properties upon pectin beads performance. Int J Pharm 321:86–93
Cheng G, An F, Zou MJ, Sun J, Hao XH, He YX (2004) Time- and pH-dependent colon-specific drug delivery for orally administered diclofenac sodium and 5-aminosalicylic acid. World J Gastroenterol 10(12):1769–1774
Costa P, Sousa LJM (2001) Modeling and comparison of dissolution profiles. Eur J Pharm Sci 13:123–133
Costa FO, Sousa JJ, Pais AA, Fornosinho SJ (2003) Comparison of dissolution profile of ibuprofen pellets. J Control Release 89:199–212
Das S, Chaudhury A (2011) Preparation and evaluation of zinc–pectin–chitosan composite particles for drug delivery to the colon: role of chitosan in modifying in vitro and in vivo drug release. Int J Pharm 406:11–20
Das S, Ng KY (2010a) Colon-specific delivery of resveratrol: optimization of multi-particulate calcium-pectinate carrier. Int J Pharm 385:20–28
Das S, Ng KY (2010b) Resveratrol-loaded calcium-pectinate beads: effects of formulation parameters on drug release and bead characteristics. J Pharm Sci 99:840–860
El-Gibaly I (2002) Oral delayed-release system based on Zn-pectinate gel (zpg) microparticles as an alternative carrier to calcium pectinate beads for colonic drug delivery. Int J Pharm 232:199–211
Frizziero L, Focherini MC, Valentini M, Reta M, Rocchi P (2002) Long term study on the efficacy and safety of lornoxicam in rheumatoid arthritis. Minerva Med 93(4):315–320
Gupta S, Aggarwal N (2007) Stomach-specific drug delivery of 5-fluorouracil using floating alginate beads. AAPS PharmSciTech 8(2):Article 48
Hagesaether E, Byeb R, Arne Sande S (2008) Ex vivo mucoadhesion of different zinc-pectinate hydrogel beads. Int J Pharm 347:9–15
Higuchi T (1963) Mechanism of sustained action medication theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J Pharm Sci 52:1145–1149
Hosny EA (1993) Polycarbophil as controlled release matrix. Int J Pharm 98(1–3):235–238
Khan MS, Sridhar BK, Srinatha A (2010) Development and evaluation of pH dependent micro beads for colon targeting. Ind J Pharm Sci 72(1):18–23
Lee CM, Kim DW, Lee HC (2004) Pectin microspheres for oral colon delivery: Preparation using spray drying method and in vitro release of indomethacin. Biotechnol Bioprocess Eng 9(3):191–195
Liu J, Zhang L, Jia Y, Hu W, Zhang J, Jiang H (2012) Preparation and evaluation of pectin-based colon-specific pulsatile capsule in vitro and in vivo. Arch Pharm Res 35(11):1927–1934
Mandal AS, Biswas N, Karim KM, Guha A, Chatterjee S, Behera M, Kuotsu K (2010) Drug delivery system based on chronobiology- A review. J Control Release 147:314–325
Mathiowitz E (1999) Oral drug delivery, small intestine and colon. In: Mathiowitz E (ed) Encyclopedia of Controlled Drug Delivery, vol 2. Wiley, New Jersey, pp 717–728
Momin M, Pundarikakshudu K (2007) Design, development and in vitro evaluation of sennosides tablets containing pectin HPMC for colonic drug delivery. Ind J Pharm Sci 68(3):394–401
Ofokansi KC, Kenechukwu FC (2013) Formulation development and evaluation of drug release kinetics from colon-targeted ibuprofen tablets based on Eudragit RL 100–chitosan interpolyelectrolyte complexes. ISRN Pharm 2013:Article 838403
Oliveira PR, Mendes C, Klein L, Sangoi M da S, Bernardi LS, Silva MA (2013) Formulation development and stability studies of norfloxacin extended-release matrix tablets. Biomed Res Int 2013:Article 716736
Orienti I, Cerchiara T, Luppi B, Bigucci F, Zuccari G, Zecchi V (2002) Influence of different chitosan salts on the release of sodium diclofenac in colon-specific delivery. Int J Pharm 238:51–59
Peppas NA (1985) Analysis of fickian and non-fickian drug release from polymers. Pharm Acta Helv 60:110–111
Philip A, Philip B (2010) Colon targeted drug delivery systems: A review on primary and novel approaches. Oman Med J 25:70–78
Ramasamy T, Subbaih Khandasamy U, Shanmugam S, Ruttala H (2012) Formulation and evaluation of chondroitin sulphate tablets of aceclofenac for colon targeted drug delivery. Iran J Pharm Res 11(2):465–479
Reza MS, Quadir MA, Haider SS (2003) Comparative evaluation of plastic hydrophobic and hydrophilic polymers as matrices for controlled-release drug delivery. J Pharm Pharmaceut Sci 6(2):274–291
Ritger PL, Peppas NA (1987) A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J Control Release 5:37–42
Rowe RC, Sheskey PJ, Owen SC (2006) Handbook of Pharmaceutical Excipients, 5th edn. Royal Pharmaceutical Society of Great Britain, London, pp 539–541
Sarfaraz MD, Hiremath D, Choudhary KPR (2010) Formulation and characterization of rifampicin microcapsules. Indian J Pharm Sci 72(1):101–105
Sinha VR, Kumria R (2001) Polysaccharides in colon-specific drug delivery. Int J Pharm 224:19–38
Sinha VR, Kumria R (2004) Polysaccharide matrices for microbially triggered drug delivery to the colon. Drug Dev Ind Pharm 30(2):143–150
Smolensky MH, Peppas NA (2007) Chronobiology, drug delivery, and chronotherapeutics. Adv Drug Delivery Rev 59:828–851
Smrdel P, Bogataj M, Zega A (2008) Shape optimization and characterization of polysaccharide beads prepared by ionotropic gelation. J Microencapsul 25(2):90–105
Sriamornsak P (1998) Investigation of pectin as a carrier for oral delivery of proteins using calcium pectinate gel beads. Int J Pharm 169:213–220
Sriamornsak P (1999) Effect of calcium concentration, hardening agent and drying condition on release characteristics of oral proteins from calcium pectinate gel beads. Eur J Pharm Sci 8:221–227
Takaya T, Niwa K, Muraoka M, Ogita I, Nagai N, Yano R, Kimura G, Yoshikawa Y, Yoshikawa H, Takada K (1998) Importance of dissolution process on systemic availability of drugs delivered by colon delivery system. J Control Release 50(1–3):111–122
Vandamme TF, Lenurry A (2002) The use of polysaccharides to target the drug to colon. Carbohydr Polym 48:219–231
Acknowledgments
This article does not contain any studies with human and animal subjects performed by any of the authors. And all authors (DJ Singhavi, AM Kamble, and S Khan) declare that they have no conflict of interest. The authors would like to thank Glenmark Pharmaceuticals Ltd. (Mumbai, India) and Lubrizol Advanced Materials Ltd. (Mumbai, India) for providing gift samples of lornoxicam and polycarbophil, respectively. Thanks are also due to Herbstreith and Fox (Germany) for giving sample of low-methoxy amidated pectin.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singhavi, D.J., Kamble, A.N. & Khan, S. Shape, optimization and in vitro evaluation of colon-specific multi-particulate system based on low-methoxy amidated pectin and polycarbophil. Journal of Pharmaceutical Investigation 44, 357–364 (2014). https://doi.org/10.1007/s40005-014-0131-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40005-014-0131-6