Abstract
Purpose
We investigated the protection offered by vaccinations and previous infections for the household transmission of Omicron variant of SARS-CoV-2.
Methods
34,666 participants of the German DigiHero cohort study with two or more household members were invited to a prospective household transmission study between June and December 2022. In case of a positive SARS-CoV-2 test in a household, symptom diaries were completed for at least 14 days. Dry blood spots (DBS) were taken from all household members at the beginning and six to eight weeks later. DBS were analyzed for SARS-CoV-2 antibodies.
Results
1191 individuals from 457 households participated. The risk of acquiring a SARS-CoV-2 infection decreased with higher S-titer levels at the time of exposure (from 80% at titer of 0 binding antibody units (BAU)/ml to 20% at titer of 3000 BAU/ml) and increased linearly with the time since vaccination/previous infection (20% for less than one month to 80% at one year). Transmission probability was also reduced when the symptoms of the primary case were mild and if preventive measures were implemented.
Conclusion
Vaccinations/previous infections offer a high protection against infection with the Omicron variant for a few months only, supporting the notion of seasonal circulation of the virus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In May 2023, the WHO declared that the COVID-19 pandemic was no longer a global health emergency [1]. SARS-CoV-2 became an endemic pathogen, and since the end of 2021, the Omicron variant dominates in many countries [2]. The Omicron variant has a higher transmissibility and has a high ability to escape immunity, while it causes more mild diseases and less hospitalizations compared to the previous variants [3]. The high transmissibility was shown in a meta-analysis in early 2022, where Madewell et al. estimated the highest secondary attack rate (SAR) for Omicron with 42.7%, followed by Alpha (36.4%) and Delta (29.7%) [4]. These estimates partly included vaccinated individuals, making a direct comparison difficult. From other research, it is known that vaccinations less effectively reduced transmission for Omicron compared to Alpha and Delta variants [5,6,7]. Similarly, a recent meta-analysis found that the protection offered by a previous infection was lower for Omicron and declined more rapidly over time compared to previous variants [8].
Besides vaccinations, less symptoms or even asymptomatic course of infection are associated with a lower risk of transmission. Furthermore, preventive measures like isolation of infected persons, mask wearing at home, disinfection, were shown to reduce the transmission of SARS-CoV-2 in households [9,10,11,12]. While several studies have already investigated the waning of protection offered by vaccination or previous infection with SARS-CoV-2, most of these studies applied a test negative design or retrospective methodology [8, 13]. There are only a few studies investigating protective antibody titers with real world data [14, 15], and they were neither done in household settings, which allows to define the timing of exposure, nor during the Omicron pandemic.
Therefore, this study aimed to determine the risk of acquiring infection from household exposure to the Omicron variant in a community setting with mixed immunity. Particularly, we wanted to estimate the effect of titer decrease and the time since last exposure (vaccination or infection) on the risk of acquiring an infection in the household.
Methods
Study design and participants
This study was conducted within the prospective cohort study for digital health research in Germany (DigiHero, DRKS Registration-ID: DRKS00025600). DigiHero is a nationwide study with currently over 90,000 participants (11/2023) and an ongoing recruitment. The study was initiated in 2021, with the overall goal to establish a digital research platform for rapid collection of health related data. DigiHero is population-based, with postal invitations sent to a random sample of persons aged 18–87 from the local registry offices in selected federal states in Germany as previously described [16]. Those interested register online and fill out a baseline questionnaire. Further questionnaires and invitations to other modules are sent out electronically three to four times per year.
Module on transmission in households
In June 2022, we initiated a substudy on the transmission of SARS-CoV-2 in the household setting among DigiHero participants, who have been recruited at this time. In total, 34,666 participants from households with two or more household members were invited. Those who expressed interest in participating in the substudy (n = 11,492) were asked to send an email to our study team within 24 h if a member of the household has been tested positive for SARS-CoV-2. Monthly reminders were sent to those with interest in participating. We defined a positive test as a positive PCR test result, one positive rapid test with symptoms, or two or more positive rapid tests without symptoms. The study team prepared a parcel with all study materials and sent it to the participants via postal mail. The parcel usually arrived the day after the study center was notified of the positive SARS-CoV-2 test. We ended the study in December 2022.
We asked the participants to collect dry blood spots from all household members using an established kit (see below) as soon as the parcel arrived at their home. All participants completed a symptom diary for the following 14 days whereby parents could fill in symptom diaries for their children. Symptoms included fever, cough, sore throat, runny nose, headache, and limb pain, tiredness, mostly lying in bed, diarrhea, vomiting, nausea, disturbance of smell or taste, insomnia, shortness of breath, and other symptoms. Participants were asked to report the intake of medications, medical consultations, stays in hospital, and tests for SARS-CoV-2. After all participants in the household recovered from their infection, we asked one person to complete an online survey about the timing and course of infections, the measures used to avoid transmission (disinfection, separate meal times, staying in separate rooms, wearing masks, keep distance, others), and previous infections and vaccinations of all household members. We sent dry blood spot kits again approximately six to eight weeks after the initial infection in the household.
The Ethics Committees of the Martin Luther University Halle-Wittenberg, Germany (No. 2020-076) approved the study. Participants received detailed information on the objective and the procedure of the study and provided written informed consent.
Laboratory analyses
Participants collected a capillary blood sample via self-sampling. For this purpose, we used a Dry Blood Spot (DBS) card from AHLSTROM-MUNKSJÖ [17]. Participants were instructed to drop the capillary blood directly from the fingertip onto the DBS cards and let it dry for at least two hours. DBS cards were stored at room temperature before analysis. Two spots with a diameter of 4.7 mm were punched per card. We discarded the DBS if it was not possible to get two soaked blood spots. DBS were eluted by addition of 500 µl Sample Buffer (Euroimmun) in deep-well plates (1 ml, Euroimmun), shaked for 30 s at 700–1000 rpm and incubated for 1 h at 37 °C. All DBS eluates were centrifuged by room temperature for 5 min and 5,000 rpm. DBS holders (Euroimmun) were put in the wells in order to fix the spots on the bottom of the well. Analysis for SARS-CoV-2 antibodies (EUROIMMUN Anti-SARS-CoV-2- QuantiVac-ELISA (IgG) & EUROIMMUN-Anti-SARS-CoV-2-NCP-ELISA (IgG)) was performed using ELISA (EUROLabWorkstation, Euroimmun). Laboratory analysis was performed by the German lab “MVZ Labor Krone eGbR”.
Definitions
In order to establish the occurrence of transmission, we combined information regarding positive tests and changes for anti-spike protein antibody titer (S-titer) and for anti-nucleocapsid protein antibody (N-titer) between the sample collected in the beginning and 6–8 weeks later. We used a stepwise approach. First, we classified all those who reported a positive SARS-CoV-2 test regardless of the type of test (rapid test or PCR) as new infections. After that, all participants with a seroconversion (change from negative to positive titer) of N- or S-titer were added. Finally, participants with a 1.5-fold increase for either N- or S- titer were considered as having been infected. We performed a sensitivity analysis in which individuals defined as infected due to the 1.5-fold increase in the antibody titers were classified as not infected. We defined individuals who first became infected in the household as index cases. If the infection of other household members started on the same day, they were classified as simultaneously infected, if there was a delay of 1–14 days they were classified as secondary cases. Households with simultaneous infections, in which there were no further uninfected household members were excluded.
To determine the symptoms of the index case, an adapted version of the acute respiratory illness (ARI) definition was used [18]. The index case was classified as having an ARI if the person (1) had fever or was so sick that she/he had to stay in bed for at least 1 day or (2) had two consecutive days with at least one respiratory symptom (cough, sore throat, cold, dyspnea) and one systemic symptom (headache, muscle ache, fatigue, or sleep disorder). If this definition was not met, the index case was either classified as mildly symptomatic (some respiratory or systemic symptoms but not meeting ARI threshold) or as asymptomatic (no symptoms reported).
Statistical analysis
We report frequencies and mean values for descriptive statistics. After defining putative transmission chains, we used generalized additive regression models to assess the association between antibody levels of the S-titer at the time-point of exposure to an infectious household member or the time since last preceding exposure (vaccination or infection) and the risk of acquiring an infection [19]. In addition, we calculated the secondary attack rate (SAR) for households, dividing secondary infections by the number of all household members. Univariable and multivariable logistic regression was used to identify factors associated with the risk of acquiring an infection.
Analysis was conducted in R (version 4.3.1) and 95% confidence intervals (CIs) are reported for all analyses.
Results
Study population and SAR
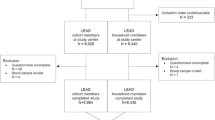
We recruited 1191 individuals from 457 households for the study. We excluded 289 household members with missing consent and/or serology at any of the two time points. 54 households were then left with only one participant and excluded. In 48 households, we could not clearly identify the occurrence of transmission because of missing dates or simultaneous infections in two person households, resulting in a final sample size of 262 households with 662 participants for the analysis of transmission (Figure S1). There were slightly more female participants, 4 out of 5 participants were adults, and two person households were most common (Table 1). Most of the household members with previous infections were also vaccinated (78%) while the proportion of previously infected among those vaccinated was lower (46%) (Table S1).
Of the 389 household members at risk, 224 were classified as secondary cases, yielding a SAR of 58% (95% CI 53–63%). Of them, 142 (63.3%) were classified as secondary cases based on a positive SARS-CoV-2 test, 24 (10.7%) based on seroconversion, and 58 (25.9%) on the 1.5-fold titer increase. The median interval between index patient onset date and household contact onset date was 3 days (IQR = 2–4 days).
We classified 168 households (64.1% of all, and 30.5% of households with more than two persons) as displaying transmission events. Most index cases (85.0%, 232 of 273) and household contacts (65.6%, 147 of 224) reported COVID-19–compatible symptoms. The majority of the household contacts with a positive SARS-CoV-2 had symptoms. The symptomatic proportions in the secondary cases were similar among those with seroconversion and titer increase (Table S2). The SAR was higher if the index case had COVID-19–compatible symptoms (59%, 95% CI 53–64%) compared to if the index case had mild symptoms (52%, 95% CI 39–65%) or no symptoms (40%, 95% CI 5–85%). Various measures were employed to reduce transmission in the households (Table S3).
Risk of acquiring infection depending on preceding vaccinations or infections
The risk of acquiring an infection from an index case was higher for those with a lower S-titer at time of exposure in the household. The association was approximately linear and similar for adults and children (Fig. 1A, B). Conversely, the risk of acquiring an infection increased similarly with the time since the last infection or vaccination (1C, D). Since we did not observe a difference between vaccinations/previous infections in the above analysis (Figures S2 and S3), we combined those.
Probability of acquiring an infection after exposure in a household in relation to the protein S-titer at the time point of exposure in adults (A, n = 285) and children (B, n = 84) and by time since last exposure (vaccination or infection, C (n = 277), D(n = 71)), censored at upper 5% to avoid unstable estimation in area of sparse data
Probability of acquiring an infection after exposure in the household
In the univariable logistic regression, the odds of acquiring an infection increased with the time since the preceding vaccination or infection (OR = 1.18, 95% CI = 1.10; 1.26 per month) (Table 2). Similar effects were found when time since vaccination was studied in those for whom vaccination was the last exposure and when time since last infection was studied among those for whom previous infection was the last exposure (Table 2), therefore both were combined. Similarly, a lower S-titer at exposure was associated with higher odds of acquiring an infection. Prevention measures applied in the household and less severe symptoms of the index case reduced the risk of infection (Table 2). There was also a difference depending on household composition with lower transmission risk when exposed were children compared to adults.
In the multivariable analysis, the estimates were similar, indicating no substantial confounding among the studied factors (Table 3). Furthermore, the results of the sensitivity analysis classifying secondary cases with a 1.5-fold titer increase as not infected or additionally including cases with change from possible to positive titer showed similar estimates (Table S4).
Discussion
In this prospective study with exposure defined by an index case in the same household, we found that the probability of acquiring an infection in the household increased almost linearly with the time since last exposure from 20% shortly after a previous exposure to over 80% at one year after the last exposure (similarly for preceding infections and vaccinations). A similar linear relationship was observed for the declining antibody S-titer. Prevention measures in the household effectively mitigated the risk.
We showed that only short times after last exposures protected household members from infection. This is in line with a previous meta-analysis showing that protection against Omicron is substantially reduced only for a short time when the past infection was with a pre-Omicron variant [8]. Even if the past infection was with Omicron, the protection was low especially for BA.4 and BA.5 which was the predominant sublineage mid 2022 in Germany [8]. Similarly, to our findings, there was an almost linear decrease of protection during the first 12 months for Omicron (only shown for BA.1). A recent meta-analysis showed that protection solely through vaccination decreased very fast and hybrid immunity had the best protection [20]. Interestingly, a previous infection seemed to provide better protection than a vaccination. However, in our analysis the probability for acquiring infection was similar for participants with last exposures being infections or vaccinations.
One interesting aspect is the situation of children in household transmission. The probability of acquiring an infection increased with the time since the last exposure also for children but not as strong as for adults. However, since the number of children included in our study was low, these results had a higher uncertainty. Children also were less likely to be the index case in our sample and less likely to transmit or acquire infection compared to the transmission among adults. Lower transmission risk was particularly documented for children for the earlier variants [21, 22], but our data indicated this effect also for the Omicron, albeit not very strong.
We also found that adherence to prevention measures in households reduced transmission. This is in line with previous research and shows that it is possible to reduce risk of infections the household setting, e.g. one study found that the isolation of the index case was associated with a lower transmission risk [23]. At the same time, the relative effect appears considerable, but the absolute effect is not very strong. We did not focus on the topic in our study and asked only very broadly about prevention measures, so the findings should be treated with caution. Low standardization and small sample size precluded analyses of individual prevention measures.
The calculated SAR in our study was high compared to previous studies reporting SAR between 31-53% [4]. First, given that transmission depends on time since the last exposure, a different composition of the sample with respect to the last vaccination or infection will affect SAR. Second, studies with denser testing are likely to identify more mild symptomatic or asymptomatic cases compared to studies with less testing. This can be related to primary as well as secondary cases. As primary cases, we included only participants who reported a positive test. Thus our primary cases are more likely to have clear symptoms prompting testing. Since the risk of transmitting infection depended on symptoms of the index case, this could have inflated the estimates. On the other side, we could have missed some secondary cases with a weak immune response, as we did not conduct systematic testing during the potential transmission phase. However, we tried to overcome this limitation by including participants with a titer seroconversion or a strong titer increase as cases. With this approach, we might also have captured asymptomatic cases but also participants who were actually not infected but just showed a strong immune reaction when being exposed. Third, for simplification, we assumed that all secondary cases in a household resulted from the index case. In fact, there could have been some infection chains, i.e. the third person was not infected by the index case, but by a secondary case. Given this aspect, SAR and risk of transmission/acquisition of an infection in our study are the upper boundary. Still the linear form of the function should not be affected by this simplification.
We showed that a household transmission study can be conducted in a relatively short time even when incidence is low. We conducted the study completely remote and showed that self-sampling of blood is feasible under pandemic conditions. In contrast to most other household transmission studies, we used a prospective approach limiting the recall bias of participants and being able to measure changes in S-titer. Participants of DigiHero are from a community setting so that mostly mild cases are captured in this study. We did not determine the SARS-CoV-2 variant, however there was a strong dominance of Omicron during the study time [24].
Our study has some limitations. It is possible that we misclassified the cases e.g. the first mild symptomatic/asymptomatic case infected the “index” which then became symptoms and was tested positive. It might be also possible that household contacts had unknown SARS-CoV-2 exposures outside from the household and were misclassified as secondary cases from the household, although they have been independently infected. Given that only adults participate in DigiHero, we may have had more households where the index case was an adult, as they may have been more likely to think about participating in the transmission module if they had tested positive themselves. We also could have missed some cases, because we did not ask for systematic testing or a proof of positive tests. DBS have been proven as a valid alternative for blood collecting, however, in some cases, we received invalid results (79 participants). There are already some self-sampling systems to collect blood in tubes and this might improve the quality of serology results in the future. Additionally, we could not consider unknown previous infections of individuals, leading to a misclassification of the previous exposure of these cases.
In conclusion, the transmissibility of the omicron variant of SARS-CoV-2 in a household exposure is high. Vaccinations and preceding infections reduce the risk of transmission only for a relatively short period, supporting the notion of seasonal circulation of the virus. Fortunately, it appears from other sources that the protection against a severe course of the SARS-CoV-2 infection lasts longer than the protection against any infection.
Data availability
The anonymized data reported in this study can be obtained from the corresponding author upon request. The dataset includes individual data and an additional data dictionary will be provided. The beginning of data availability starts with the date of publication and the authors will support any requests in the three following years. Data requests should include a proposal for the planned analyses. Decisions will be made according to data use by the access committee of the DigiHero study, and data transfer will require a signed data access agreement.
References
Statement on the fifteenth meeting of the IHR (2005) Emergency Committee on the COVID-19 pandemic. 2/7/2024. https://www.who.int/news/item/05-05-2023-statement-on-the-fifteenth-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-coronavirus-disease-(covid-19)-pandemic. Accessed 7 Jun 2024
One year since the emergence of COVID-19 virus variant Omicron. 2/8/2024. https://www.who.int/news-room/feature-stories/detail/one-year-since-the-emergence-of-omicron. Accessed 7 Jun 2024.
Mohsin M, Mahmud S. Omicron SARS-CoV-2 variant of concern: a review on its transmissibility, immune evasion, reinfection, and severity. Medicine (Baltimore). 2022;101: e29165. https://doi.org/10.1097/MD.0000000000029165.
Madewell ZJ, Yang Y, Longini IM, Halloran ME, Dean NE. Household secondary attack rates of SARS-CoV-2 by variant and vaccination status: an updated systematic review and meta-analysis. JAMA Netw Open. 2022;5: e229317. https://doi.org/10.1001/jamanetworkopen.2022.9317.
Lyngse FP, Kirkeby CT, Denwood M, Christiansen LE, Mølbak K, Møller CH, et al. Household transmission of SARS-CoV-2 Omicron variant of concern subvariants BA.1 and BA.2 in Denmark. Nat Commun. 2022;13:5760. https://doi.org/10.1038/s41467-022-33498-0.
Jalali N, Brustad HK, Frigessi A, MacDonald EA, Meijerink H, Feruglio SL, et al. Increased household transmission and immune escape of the SARS-CoV-2 Omicron compared to Delta variants. Nat Commun. 2022;13:5706. https://doi.org/10.1038/s41467-022-33233-9.
Tenforde MW, Kim SS, Lindsell CJ, Billig Rose E, Shapiro NI, Files DC, et al. Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network - United States, March–June 2020. MMWR Morb Mortal Wkly Rep. 2020;69:993–8. https://doi.org/10.15585/mmwr.mm6930e1.
Caroline Stein, Hasan Nassereldine, Reed J D Sorensen, Joanne O Amlag, Catherine Bisignano, Sam Byrne, et al. Past SARS-CoV-2 infection protection against re-infection: a systematic review and meta-analysis. Lancet. 2023;401:833–42. https://doi.org/10.1016/S0140-6736(22)02465-5.
Baker JM, Nakayama JY, O’Hegarty M, McGowan A, Teran RA, Bart SM, et al. SARS-CoV-2 B.1.1.529 (Omicron) Variant Transmission Within Households - Four U.S. Jurisdictions, November 2021-February 2022. MMWR. Morbidity and mortality weekly report 2022, 71: 341–346. https://doi.org/10.15585/mmwr.mm7109e1.
Liu Y, Xu L, Piao X, Li H, Shi L, Huang Y, et al. Epidemiological, clinical, and household transmission characteristics of children and adolescents infected with SARS-CoV-2 Omicron variant in Shanghai, China: a retrospective, multicenter observational study. Int J Infect Dis. 2023;129:1–9. https://doi.org/10.1016/j.ijid.2023.01.030.
Wang Y, Tian H, Zhang L, Zhang M, Guo D, Wu W, et al. Reduction of secondary transmission of SARS-CoV-2 in households by face mask use, disinfection and social distancing: a cohort study in Beijing. China BMJ Glob Health. 2020. https://doi.org/10.1136/bmjgh-2020-002794.
Goodwin L, Hayward T, Krishan P, Nolan G, Nundy M, Ostrishko K, et al. Which factors influence the extent of indoor transmission of SARS-CoV-2? A rapid evidence review. J Glob Health. 2021;11:10002. https://doi.org/10.7189/jogh.11.10002.
Menegale F, Manica M, Zardini A, Guzzetta G, Marziano V, d’Andrea V, et al. Evaluation of waning of SARS-CoV-2 Vaccine-Induced immunity: a systematic review and meta-analysis. JAMA Netw Open. 2023;6: e2310650. https://doi.org/10.1001/jamanetworkopen.2023.10650.
Sullivan A, Alfego D, Hu P, Gillim L, Grover A, Garcia C, et al. Antibody titer levels and the effect on subsequent SARS-CoV-2 infection in a large US-based cohort. Heliyon. 2023;9: e13103. https://doi.org/10.1016/j.heliyon.2023.e13103.
Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–11. https://doi.org/10.1038/s41591-021-01377-8.
Gottschick C, Diexer S, Massag J, Klee B, Broda A, Purschke O, et al. Mental health in Germany in the first weeks of the Russo-Ukrainian war. BJPsych Open. 2023;9: e66. https://doi.org/10.1192/bjo.2023.21.
Ahlstrom. Biosamples collection cards. 2/8/2024. https://www.ahlstrom.com/products/medical-life-sciences-and-laboratory/specimen-collection-cards/Biosamples-collection-cards/. Accessed 7 Jun 2024.
Verberk JDM, de Hoog MLA, Westerhof I, van Goethem S, Lammens C, Ieven G, et al. Transmission of SARS-CoV-2 within households: a remote prospective cohort study in European countries. Eur J Epidemiol. 2022;37:549–61. https://doi.org/10.1007/s10654-022-00870-9.
Comprehensive R Archive Network (CRAN). mgcv: Mixed GAM Computation Vehicle with Automatic Smoothness Estimation. 11/28/2023. https://cran.r-project.org/web/packages/mgcv/index.html. Accessed 7 Jun 2024.
Bobrovitz N, Ware H, Ma X, Li Z, Hosseini R, Cao C, et al. Protective effectiveness of previous SARS-CoV-2 infection and hybrid immunity against the omicron variant and severe disease: a systematic review and meta-regression. Lancet Infect Dis. 2023;23:556–67. https://doi.org/10.1016/S1473-3099(22)00801-5.
Silverberg SL, Zhang BY, Li SNJ, Burgert C, Shulha HP, Kitchin V, et al. Child transmission of SARS-CoV-2: a systematic review and meta-analysis. BMC Pediatr. 2022;22:172. https://doi.org/10.1186/s12887-022-03175-8.
Chen F, Tian Y, Zhang L, Shi Y. The role of children in household transmission of COVID-19: a systematic review and meta-analysis. Int J Infect Dis. 2022;122:266–75. https://doi.org/10.1016/j.ijid.2022.05.016.
Inaba M, Miyake Y, Yasuda K. Secondary household transmission of SARS-CoV-2: a case-control study on factors associated with reduced transmission risk. Int J Infect Dis. 2023;137:4–8. https://doi.org/10.1016/j.ijid.2023.09.019.
Robert Koch Institut. Wöchentlicher COVID-19 Lagebericht vom 12.01.2023, https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Situationsberichte/Wochenbericht/Wochenbericht_2023-01-12.pdf?__blob=publicationFile. Accessed 27 June 2024.
Acknowledgements
We thank all DigiHero participants for their efforts and contributions and Mareike Kunze for excellent technical assistance.
Funding
Open Access funding enabled and organized by Projekt DEAL. The DigiHero study is supported by internal resources of the Medical Faculty of the Martin Luther University Halle-Wittenberg. The transmission study was supported by the Ministry of Economy, Science, and Digitalization of the Federal State of Saxony-Anhalt, Germany [CoPreP, grant number CSV 8].
Author information
Authors and Affiliations
Contributions
RM, BK, SD, CG and CH developed the module. BK, SD, CX and RM wrote the original draft of the manuscript. RM, BK, SD, and CX contributed to the literature review. BK and SD performed the data analysis. BK was responsible for data curation. BK, SD, CG and CH contributed to data collection. KMS contributed to analysis and interpretation of laboratory results. BK, SD, CX, CG, CH, KMS, SM, JR, MB, TF, MG, MG, JH, DS, AK and RM contributed to reviewing and editing of the manuscript. RM provided supervision of the original study and supervision of the analysis. BK and SD directly accessed and verified the underlying data reported in the manuscript. All authors accepted the final version of the manuscript and took responsibility for the decision to submit for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval
The Ethics Committee of the Martin Luther University Halle-Wittenberg (2020–076) approved the study. Informed consent was obtained from all participants.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Klee, B., Diexer, S., Xu, C. et al. Household transmission of Omicron variant of SARS-CoV-2 under conditions of hybrid immunity—a prospective study in Germany. Infection (2024). https://doi.org/10.1007/s15010-024-02352-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s15010-024-02352-4