Abstract
Objective
Carbapenem-resistant Enterobacteriaceae (CRE) pose a significant threat to human health and have emerged as a major public health concern. We aimed to compare the efficacy and the safety of ceftazidime–avibactam (CAZ–AVI) and polymyxin in the treatment of CRE infections.
Methods
A systematic review and meta-analysis was performed by searching the databases of EMBASE, PubMed, and the Cochrane Library. Published studies on the use of CAZ–AVI and polymyxin in the treatment of CRE infections were collected from the inception of the database until March 2023. Two investigators independently screened the literature according to the inclusion and exclusion criteria, evaluated the methodological quality of the included studies and extracted the data. The meta-analysis was performed using RevMan 5.4 software.
Results
Ten articles with 833 patients were included (CAZ–AVI 325 patients vs Polymyxin 508 patients). Compared with the patients who received polymyxin-based therapy, the patients who received CAZ–AVI therapy had significantly lower 30-days mortality (RR = 0.49; 95% CI 0.01–2.34; I2 = 22%; P < 0.00001), higher clinical cure rate (RR = 2.70; 95% CI 1.67–4.38; I2 = 40%; P < 0.00001), and higher microbial clearance rate (RR = 2.70; 95% CI 2.09–3.49; I2 = 0%; P < 0.00001). However, there was no statistically difference in the incidence of acute kidney injury between patients who received CAZ–AVI and polymyxin therapy (RR = 1.38; 95% CI 0.69–2.77; I2 = 22%; P = 0.36). In addition, among patients with CRE bloodstream infection, those who received CAZ–AVI therapy had significantly lower mortality than those who received polymyxin therapy (RR = 0.44; 95% CI 0.27–0.69, I2 = 26%, P < 0.00004).
Conclusions
Compared to polymyxin, CAZ–AVI demonstrated superior clinical efficacy in the treatment of CRE infections, suggesting that CAZ–AVI may be a superior option for CRE infections.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent decades, carbapenem-resistant Enterobacteriaceae (CRE) have been identified by the World Health Organization as a formidable medical threat to public health [1, 2]. Infections caused by CRE resulted in extremely high morbidity and mortality, and recent studies have revealed a mortality rate exceeding 65% for bloodstream infections (BSIs) caused by CRE [3]. Despite the severity of CRE infections, the treatment options for CRE infections are still very limited. Currently, carbapenems (meropenem, imipenem), polymyxins, tigecycline, aminoglycosides, and ceftazidime–avibactam (CAZ–AVI) are the main antibiotics used to treat CRE infections [4, 5]. Based on reports of in vitro activity and clinical efficacy, polymyxins has been used as a first-line agent to treat CRE infections [6, 7].
The polymyxins currently in clinical use include colistin and polymyxin B [8]. However, due to its toxicity (such as nephrotoxicity and neurotoxicity), limited efficacy, dose uncertainty (suboptimal pharmacokinetic dose), heterogeneous resistance mediated by mcr-1, and the limited accuracy of in vitro susceptibility testing, polymyxin cannot be utilized as a last resort for the treatment of CRE infections [6, 9,10,11,12].
In 2015, CAZ–AVI was approval by the U.S. Food and Drug Administration (FDA) for the treatment of complicated abdominal infections (cIAIs), complicated urinary tract infections (cUTIs), as well as hospital-acquired, and ventilator-associated pneumonia (HAP/VAP) [13]. In 2019, CAZ–AVI was approved in China as the only new β-lactam/β-lactamase inhibitor combinations for the treatment of cIAI, HAP, and VAP caused by multi-drug-resistant Gram-negative bacteria. Preliminary evidence suggests that CAZ–AVI-based regimens are more effective than current treatments for CRE infections [14,15,16].
Due to the limited availability of data, previous meta-analyses on the efficacy of CAZ-AVI in treating CRE infections usually paid little attention to the comparison of CAZ–AVI with specific antibacterial agents. Meta-analyses have been conducted to compare the efficacy of CAZ–AVI with other available antibacterial agents, including the mix of carbapenems, polymyxins, tigecycline, and aminoglycosides [17, 18]. However, no meta-analysis has been conducted to compare the efficacy of CAZ-AVI and polymyxins in treating CRE infection, and most of the real-world studies comparing antibacterial agents for the treatment of CRE have been small sample sizes. The aim of this meta-analysis was therefore to compare the efficacy of CAZ–AVI-based therapy with polymyxin-based therapy in the treatment of CRE infections.
Methods
Search strategy
This study was conducted according to PRISMA statement. A systematic search was conducted across three databases, namely PubMed, EMBASE, and the Cochrane Library. Additionally, a manual search of references were performed to identify included literature. The search period spans from the inception of database construction to March 18, 2023. The search terms utilized were “carbapenem-resistant Enterobacteriaceae”, “avibactam-ceftazidime”, and “polymyxins”. Subject headings and free texts (i.e., Medical Subject Headings [MeSH] terms) were identified for the search terms. The complete search strategies are provided in the Supplementary Material.
Selection criteria
Two investigators independently conducted the literature search and screened the literature. Studies were eligible for inclusion if they (1) compared the efficacy of CAZ-AVI and polymyxins in patients with CRE infections, (2) reported primary outcome (30-days mortality), and (3) were prospective/retrospective observational cohort, case–control studies, or randomized controlled trials (RCTs), Exclusion criteria were (1) studies not published in English, (2) studies that did not provide adequate information.
Quality assessment
The literature's quality was independently evaluated by two investigators. The methodological quality of cohort or case–control studies was assessed using the Newcastle–Ottawa Scale (NOS), including risk of bias in patient selection, comparability between groups, and exposure or outcome. Studies with NOS scores ≥ 7 were high quality studies. The methodological quality of included RCTs was assessed using the Cochrane Collaboration ‘risk of bias’ tool.
Data extraction
The following information was extracted from the included studies: (1) first author and publication year, (2) study characteristics including design, duration, and sample size, (3) patient characteristics including infection type and pathogen, and (4) clinical outcomes: 30-days mortality rate, clinical cure rate, microbial clearance rate, and adverse effects rate.
Definitions
The primary outcome of this study was 30-days mortality including 28-days mortality, while the secondary outcomes were clinical cure and microbial clearance. Clinical cure was defined as the resolution of clinical signs and symptoms of infection, as documented by the clinician, along with follow-up data indicating that the patient has achieved microbiological eradication [19]. Microbial clearance was defined as negative CRE culture after antibacterial therapy. CAZ–AVI-based therapy was defined as treatment with CAZ–AVI or in combination with other antibacterial agents. Polymyxin-based therapy was defined as treatment with polymyxin or in combination with other antimicrobial agents other than CAZ–AVI [20].
Statistical analysis
Meta-analysis was performed using Review Manager 5.3 statistical software provided by the Cochrane Collaboration network. All outcome indicators in this study were dichotomous variables, so relative risk (RRs) and 95% confidence interval (CI) were used to express them. Testing for heterogeneity using the I2 statistic, random effects models were used for results with high heterogeneity (I2 > 50%), while fixed effects models were used when heterogeneity was not significant. Inverted Funnel plot was used to detect publication bias, and sensitivity analysis was used to determine the robustness of the results of this analysis.
Results
Study selection
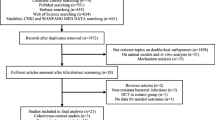
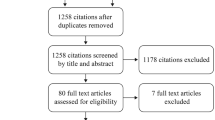
The search strategy developed in this study yielded 510 references through electronic database search and an additional 4 references were identified via reference list. There were 394 references after removing duplicates, and 340 references were excluded after reading the abstract and title. Of the 54 available literatures, eleven were excluded due to lack of reported primary outcomes, 24 were excluded because they did not include polymyxin in the control group, and 9 were excluded due to unavailability of important data. Finally, ten references (nine full paper and one conference paper) were included in this study for meta-analysis. Figure 1 listed the flow diagram of included studies.
Study characteristics
The characteristics of the included ten studies are listed in Table 1. In ten studies [21,22,23,24,25,26,27,28,29,30], all were observational studies, eight of which were retrospective, and two of which were prospective, including two case–control, eight cohort studies. Among the ten studies, six were multicenter studies, and four were single-center studies. The sample size ranged from 32 to 164. The included ten studies enrolled 833 patients with 325 receiving the CAZ–AVI therapy and 508 receiving the polymyxin therapy. Table 1 lists the specific antimicrobial regimens for each study and their effects on 30-day mortality. All ten studies reported 30-day mortality (or 28-day mortality), three studies reported clinical cure rates, two studies reported microbial clearance, and three studies reported acute kidney injury. The most frequently investigated pathogen was CRKP, followed by CR and CPE. The primary infections were BSIs, followed by any infection (Table 1). The scoring details are shown in Table 2. The NOS scores of ten studies were ≥ 7 scores (Table 2).
Results of meta-analysis
All 10 studies reported 30-day mortality. Compared with the patients who received polymyxin-based therapy, the patients who received CAZ–AVI therapy had significantly lower 30-day mortality (RR = 0.49; 95% CI 0.01–2.34; I2 = 22%; P < 0.00001; Fig. 2). Subgroup analysis showed that 30-day mortality was significantly lower in patients treated with CAZ–AVI than in patients treated with polymyxin B (RR = 0.50; 95% CI 0.38 ~ 0.64; I2 = 0%; P < 0.00001; Fig. 2), while 30-day mortality was lower in patients treated with CAZ–AVI than in patients treated with colistin, but there was no statistical difference (RR = 0.49; 95% CI 0.21 ~ 1.14; I2 = 65%; P < 0.10; Fig. 2).
Compared with the patients who received polymyxin-based therapy, the patients who received CAZ–AVI therapy had significantly higher clinical cure rate (RR = 2.70; 95% CI 1.67 ~ 4.38; I2 = 40%; P < 0.00001; Fig. 3), and higher microbial clearance rate (RR = 2.70; 95% CI 2.09 ~ 3.49; I2 = 0%; P < 0.00001, Fig. 4). In addition, among patients with CRE bloodstream infection, those who received CAZ–AVI therapy had significantly lower mortality than those who received polymyxin therapy (RR = 0.44; 95% CI 0.27 ~ 0.69, I2 = 26%, P < 0.00004; Fig. 5). However, there was no statistically difference in the incidence of acute kidney injury between patients who received CAZ–AVI and polymyxin therapy (RR = 1.38; 95% CI 0.69 ~ 2.77; I2 = 22%; P = 0.36; Fig. 6).
Sensitivity analysis
Sensitivity analysis was performed by sequentially excluding studies in this meta-analysis. The finally results demonstrated no significant alterations in RR, P, and I2 outcomes following the exclusion of each study. The sensitivity analysis did not have an impact on the 30-day mortality, clinical cure rate, microbial clearance, and incidence of acute kidney injury, which indicates that the results of this meta-analysis have certain robustness.
Discussion
The options for treating CRE infections with antibiotics are extremely limited. In the clinical management of CRE infection, the selection of appropriate antibiotics has always posed a formidable challenge. Early selection of appropriate active antibiotics following infection with CRE is crucial for reducing mortality rates and improving clinical outcomes. According to the Infectious Diseases Society of America (IDSA) and the European Society for Clinical Microbiology and Infectious Diseases (ESCMID), the optimal antimicrobial regimen for treating CRE infections remains undetermined [4, 5].
The results of this meta-analysis indicated CAZ–AVI-based therapy was significantly superior to polymyxin-based therapy. Furthermore, the findings of this study demonstrate that CAZ–AVI outperforms polymyxin in terms of the primary outcome for patients with CRE-BSI.
Prior to the introduction of new drugs, such as CAZ–AVI, polymyxin was frequently utilized in both monotherapy and combination regimens. However, the following limitations limit the use of polymyxins: (1) Polymyxin exhibits high incidence of adverse reactions, particularly nephrotoxicity and neurotoxicity, (2) Polymyxins are antibacterial drugs that exhibit concentration-dependent activity and have a relatively narrow therapeutic window. For instance, polymyxin E achieves an effective steady-state blood concentration of 2 mg/L, with the risk of nephrotoxicity increasing at concentrations exceeding 2.3 mg/L—a range that almost overlaps with the threshold for toxicity, (3) Both colistin and polymyxin B can induce drug resistance during treatment; thus, combination therapy is recommended for severe infections, (4) It is difficult to reach the required concentration of the drug in lung tissue and body fluids during intravenous administration of polymyxin, and the PK/PD target achievement rate in patients with pulmonary infection is significantly decreased [10,11,12]. The results of this study indicate that there was no significant difference in the incidence of severe kidney injury between patients treated with polymyxin and those treated with CAZ–AVI. This may be attributed to insufficient attention paid to the comparison of nephrotoxicity between CAZ–AVI and polymyxin, resulting in inadequate sample size and inaccurate findings.
In recent years, CAZ–AVI, as a new antibacterial combination, was approved by the US FDA for the treatment of CRE infection in 2015. Studies have analyzed the effect of CAZ–AVI-based therapy on adverse outcomes in patients with CRE infections. The study findings indicated that there was no statistically significant difference in mortality rates between patients who received combination therapy with CAZ–AVI and those who received monotherapy [16, 30, 31]. This conclusion was further supported by two separate meta-analyses [32, 33]. Similarly, many studies have compared the efficacy of CAZ–AVI with other antibacterial agents, including carbapenems, tigecycline, and polymyxin for treating infections caused by CRE. The results showed a higher mortality rate in patients treated with other antimicrobial agents [15, 16, 28, 31, 34]. This finding was also supported by several meta-analyses [17, 18, 35]. CAZ-AVI in combination with another in vitro-sensitive antimicrobial agent, including carbapenems, fosfomycin, or tigecycline, significantly reduced 30-day mortality in critically ill patients with CRE infections [14]. However, a larger sample size is required to validate this conclusion and to identify more optimal antimicrobial agents for combination therapy regimens. In short, preliminary evidence suggests a potential role for CAZ–AVI in patients with CRE infection.
However, the gradual increase in resistance to CAZ–AVI has resulted in reduced efficacy due to β-lactamase production, efflux pump activity and target modification [36]. In addition to CAZ–AVI, other novel antibiotics for the treatment of CRE infections have been approved or are in advanced clinical development, including ceftolozane–tazobactam, meropenem–vaborbactam, and imipenem–cilastatin–relebactam [37]. Meropenem–vaborbactam has demonstrated promising outcomes in treating CRE infections in the TANGO II clinical trial. However, given its limited sample size, further clinical studies are necessary to evaluate both its efficacy and safety. Due to limited data on these novel antibiotics, we did not compare the efficacy of these antimicrobials to that of CAZ–AVI or other antimicrobials. As the prevalence of drug-resistant continues to escalate, there is an urgent need for the development of novel therapeutics to combat infections caused by CRE.
This study has several limitations, first, the included studies were observational studies with small sample sizes and no randomized controlled trials (RCTs), which inevitably introduces confounding factors and bias; Second, the heterogeneity of colistin was found to be greater in the subgroup analysis. The study design and the pathogens were identified as potential reasons for this variability; Third, only three studies have reported data on the nephrotoxicity of CAZ–AVI and polymyxin, with no other adverse reactions such as neurotoxicity or cutaneous adverse reactions being reported. Finally, due to limited data, this study did not control for other confounding factors (such as the severity of patients’ infections, underlying diseases, etc.).
Conclusions
The meta-analysis compared the efficacy and the safety of CAZ–AVI and polymyxins in the treatment of CRE infections. Compared to polymyxins, CAZ–AVI demonstrated superior clinical efficacy in the treatment of CRE infections, suggesting that CAZ–AVI may be a superior option for CRE infections. In addition, there was no significant difference in safety between the two treatment options.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article [and/or supplementary materials].
References
Munoz-Price LS, Poirel L, Bonomo RA, Schwaber MJ, Daikos GL, Cormican M, Cornaglia G, Garau J, Gniadkowski M, Hayden MK, et al. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect Dis. 2013;13:785–96.
Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet DL, Pulcini C, Kahlmeter G, Kluytmans J, Carmeli Y, et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18:318–27.
Li X, Ye H. Clinical and Mortality Risk Factors in Bloodstream Infections with Carbapenem-Resistant Enterobacteriaceae. Can J Infect Dis Med Microbiol. 2017;2017:6212910.
Tamma PD, Aitken SL, Bonomo RA, Mathers AJ, van Duin D, Clancy CJ. Infectious Diseases Society of America guidance on the treatment of Extended-spectrum beta-lactamase producing Enterobacterales (ESBL-E), carbapenem-resistant Enterobacterales (CRE), and Pseudomonas aeruginosa with difficult-to-treat resistance (DTR-P. aeruginosa). Clin Infect Dis. 2021;72:e169–83.
Paul M, Carrara E, Retamar P, Tangden T, Bitterman R, Bonomo RA, de Waele J, Daikos GL, Akova M, Harbarth S, et al. European Society of Clinical Microbiology and Infectious Diseases (ESCMID) guidelines for the treatment of infections caused by multidrug-resistant Gram-negative bacilli (endorsed by European society of intensive care medicine). Clin Microbiol Infect. 2022;28:521–47.
Morrill HJ, Pogue JM, Kaye KS, LaPlante KL. Treatment options for carbapenem-resistant Enterobacteriaceae infections. Open Forum Infect Dis. 2015;2: ofv050.
Tompkins K, van Duin D. Treatment for carbapenem-resistant Enterobacterales infections: recent advances and future directions. Eur J Clin Microbiol Infect Dis. 2021;40:2053–68.
Liu Y, Yu Y, Li J, Shi Y. Multi-disciplinary expert consensus on the optimal clinical use of the polymyxins in China. Zhonghua Jie He He Hu Xi Za Zhi. 2021;44:292–310.
Matuschek E, Åhman J, Webster C, Kahlmeter G. Antimicrobial susceptibility testing of colistin—evaluation of seven commercial MIC products against standard broth microdilution for Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Acinetobacter spp. Clin Microbiol Infect. 2018;24:865–70.
Tsuji BT, Pogue JM, Zavascki AP, Paul M, Daikos GL, Forrest A, Giacobbe DR, Viscoli C, Giamarellou H, Karaiskos I, et al. International consensus guidelines for the optimal use of the polymyxins: endorsed by the American College of Clinical Pharmacy (ACCP), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases Society of America (IDSA), International Society for Anti-infective Pharmacology (ISAP), Society of Critical Care Medicine (SCCM), and Society of Infectious Diseases Pharmacists (SIDP). Pharmacotherapy. 2019;39:10–39.
Oliota AF, Penteado ST, Tonin FS, Fernandez-Llimos F, Sanches AC. Nephrotoxicity prevalence in patients treated with polymyxins: a systematic review with meta-analysis of observational studies. Diagn Microbiol Infect Dis. 2019;94:41–9.
Almangour TA, Garcia E, Zhou Q, Forrest A, Kaye KS, Li J, Velkov T, Rao GG. Polymyxins for the treatment of lower respiratory tract infections: lessons learned from the integration of clinical pharmacokinetic studies and clinical outcomes. Int J Antimicrob Agents. 2021;57: 106328.
Dietl B, Martínez LM, Calbo E, Garau J. Update on the role of ceftazidime-avibactam in the management of carbapenemase-producing Enterobacterales. Future Microbiol. 2020;15:473–84.
Zheng G, Zhang J, Wang B, Cai J, Wang L, Hou K, Zhang Y, Zhang L, Yang Z, He J, et al. Ceftazidime-avibactam in combination with in vitro non-susceptible antimicrobials versus ceftazidime-avibactam in monotherapy in critically ill patients with carbapenem-resistant Klebsiella pneumoniae infection: a retrospective cohort study. Infect Dis Ther. 2021;10:1699–713.
Gu J, Xu J, Zuo TT, Chen YB. Ceftazidime-avibactam in the treatment of infections from carbapenem-resistant Klebsiella pneumoniae: Ceftazidime-avibactam against CR-KP infections. J Glob Antimicrob Resist. 2021;26:20–5.
Tumbarello M, Raffaelli F, Giannella M, Mantengoli E, Mularoni A, Venditti M, De Rosa FG, Sarmati L, Bassetti M, Brindicci G, et al. Ceftazidime-avibactam use for Klebsiella pneumoniae carbapenemase-producing K. Pneumoniae infections: a retrospective observational multicenter study. Clin Infect Dis. 2021;73:1664–76.
Sternbach N, Leibovici Weissman Y, Avni T, Yahav D. Efficacy and safety of ceftazidime/avibactam: a systematic review and meta-analysis. J Antimicrob Chemother. 2018;73:2021–9.
Chen Y, Huang HB, Peng JM, Weng L, Du B. Efficacy and safety of ceftazidime-avibactam for the treatment of carbapenem-resistant Enterobacterales bloodstream infection: a systematic review and meta-analysis. Microbiol Spectr. 2022;10: e0260321.
Bassetti M, Giacobbe DR, Patel N, Tillotson G, Massey J. Efficacy and safety of meropenem-vaborbactam versus best available therapy for the treatment of carbapenem-resistant Enterobacteriaceae infections in patients without prior antimicrobial failure: a post hoc analysis. Adv Ther. 2019;36:1771–7.
Shen L, Lian C, Zhu B, Yao Y, Yang Q, Zhou J, Zhou H. Bloodstream infections due to carbapenem-resistant Kebsiella pneumoniae: a single-center retrospective study on risk factors and therapy options. Microb Drug Resist (Larchmont, NY). 2021;27:227–33.
Zhou C, Jin L, Wang Q, Wang X, Chen F, Gao Y, Zhao C, Chen H, Cao B, Wang H. Bloodstream infections caused by carbapenem-resistant Enterobacterales: risk factors for mortality, antimicrobial therapy and treatment outcomes from a prospective multicenter study. Infect Drug Resist. 2021;14:731–42.
Zheng G, Cai J, Zhang L, Chen D, Wang L, Qiu Y, Deng H, Bai H, Bian X, He J. Ceftazidime/avibactam-based versus polymyxin B-based therapeutic regimens for the treatment of carbapenem-resistant klebsiella pneumoniae infection in critically ill patients: a retrospective cohort study. Infect Dis Ther. 2022;11:1917–34.
Shields RK, Nguyen MH, Chen L, Press EG, Potoski BA, Marini RV, Doi Y, Kreiswirth BN, Clancy CJ. Ceftazidime-avibactam is superior to other treatment regimens against carbapenem-resistant Klebsiella pneumoniae bacteremia. Antimicrob Agents Chemother. 2017. https://doi.org/10.1128/AAC.00883-17.
Satlin MJ, Chen L, Gomez-Simmonds A, Marino J, Weston G, Bhowmick T, Seo SK, Sperber SJ, Kim AC, Eilertson B, et al. Impact of a rapid molecular test for Klebsiella pneumoniae carbapenemase and ceftazidime-avibactam use on outcomes after bacteremia caused by carbapenem-resistant Enterobacterales. Clin Infect Dis. 2022. https://doi.org/10.1093/cid/ciac354.
Meng H, Han L, Niu M, Xu L, Xu M, An Q, Lu J. Risk factors for mortality and outcomes in hematological malignancy patients with carbapenem-resistant Klebsiella pneumoniae bloodstream infections. Infect Drug Resist. 2022;15:4241–51.
John J, Nelson B. Ceftazidime-avibactam vs. Polymyxin B in the treatment of infections due to carbapenem-resistant enterobacteriaceae. Open Forum Infect Dis. 2019. https://doi.org/10.1093/ofid/ofz360.1940.
Hakeam HA, Alsahli H, Albabtain L, Alassaf S, Al Duhailib Z, Althawadi S. Effectiveness of ceftazidime-avibactam versus colistin in treating carbapenem-resistant Enterobacteriaceae bacteremia. Int J Infect Dis. 2021;109:1–7.
Fang J, Li H, Zhang M, Shi G, Liu M, Wang Y, Bian X. Efficacy of ceftazidime-avibactam versus polymyxin B and risk factors affecting clinical outcomes in patients with carbapenem-resistant Klebsiella pneumoniae infections a retrospective study. Front Pharmacol. 2021;12: 780940.
Falcone M, Daikos GL, Tiseo G, Bassoulis D, Giordano C, Galfo V, Leonildi A, Tagliaferri E, Barnini S, Sani S, et al. Efficacy of Ceftazidime-avibactam Plus Aztreonam in Patients With Bloodstream Infections Caused by Metallo-beta-lactamase-Producing Enterobacterales. Clin Infect Dis. 2021;72:1871–8.
Chen L, Han X, Li Y, Li M. Assessment of mortality-related risk factors and effective antimicrobial regimens for treatment of bloodstream infections caused by carbapenem-resistant Enterobacterales. Antimicrob Agents Chemother. 2021;65: e0069821.
Tumbarello M, Trecarichi EM, Corona A, De Rosa FG, Bassetti M, Mussini C, Menichetti F, Viscoli C, Campoli C, Venditti M, et al. Efficacy of ceftazidime-avibactam salvage therapy in patients with infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae. Clin Infect Dis. 2019;68:355–64.
Fiore M, Alfieri A, Di Franco S, Pace MC, Simeon V, Ingoglia G, Cortegiani A. Ceftazidime-avibactam combination therapy compared to ceftazidime-avibactam monotherapy for the treatment of severe infections due to carbapenem-resistant pathogens: a systematic review and network meta-analysis. Antibiotics (Basel). 2020;9:338.
Onorato L, Di Caprio G, Signoriello S, Coppola N. Efficacy of ceftazidime/avibactam in monotherapy or combination therapy against carbapenem-resistant Gram-negative bacteria: a meta-analysis. Int J Antimicrob Agents. 2019;54:735–40.
Van Duin D, Lok JJ, Earley M, Cober E, Richter SS, Perez F, Salata RA, Kalayjian RC, Watkins RR, Doi Y, et al. Colistin versus ceftazidime-avibactam in the treatment of infections due to carbapenem-resistant Enterobacteriaceae. Clin Infect Dis. 2018;66:163–71.
Chen M, Zhang M, Huang P, Lin Q, Sun C, Zeng H, Deng Y. Novel β-lactam/β-lactamase inhibitors versus alternative antibiotics for the treatment of complicated intra-abdominal infection and complicated urinary tract infection: a meta-analysis of randomized controlled trials. Expert Rev Anti Infect Ther. 2018;16:111–20.
Wang Y, Wang J, Wang R, Cai Y. Resistance to ceftazidime-avibactam and underlying mechanisms. J Glob Antimicrob Resist. 2020;22:18–27.
Doi Y. Treatment options for carbapenem-resistant Gram-negative bacterial infections. Clin Infect Dis. 2019;69:S565–75.
Funding
This study was supported by grants from the Natural Science Foundation of Hunan Province, China (2022JJ30922), and the Program of Natural Science Foundation of Hunan Province, China (2022JJ80045).
Author information
Authors and Affiliations
Contributions
Conceptualization, SD; methodology, QH and JC; formal analysis, JC; investigation, JC and QH; resources, QH and SD; data curation, JC and QH; writing-original draft preparation, JC; writing-review and editing, QH and PZ; supervision, QH, SD, and PZ; funding acquisition, SD All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declared no conflict of interest.
Consent for publication
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, J., Hu, Q., Zhou, P. et al. Ceftazidime–avibactam versus polymyxins in treating patients with carbapenem-resistant Enterobacteriaceae infections: a systematic review and meta-analysis. Infection 52, 19–28 (2024). https://doi.org/10.1007/s15010-023-02108-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-023-02108-6