Abstract
Purpose
Raising awareness of respiratory diphtheria and for the importance of early antitoxin administration.
Methods
Report of a case of fulminant, imported respiratory diphtheria in an otherwise healthy 24-year-old Afghan refugee in Austria in May 2022.
Result
This was the first case of respiratory diphtheria in Austria since 1993. Diphtheria antitoxin was administered at an already progressed disease stage. This delay contributed to a fulminant disease course with multiorgan failure and death.
Conclusion
In high-income countries with low case numbers, awareness of respiratory diphtheria and for the importance of early antitoxin administration must be raised.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the development of the diphtheria antitoxin in 1890 [1] as well as the diphtheria toxoid vaccine in the early 1920s [2], cases of respiratory diphtheria became rare in Europe. The incidence in the WHO European region has declined until 2011. In the last decade the number of cases undulated between 32 and 73 cases per year of respiratory and cutaneous diphtheria [3]. The present case has been the first case of respiratory diphtheria in Austria for the last 29 years [4].
Case report
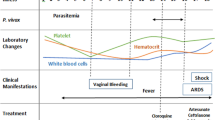
On 23rd May 2022, a 24-year-old Afghan refugee accommodated in an Austrian refugee center presented to the ear nose, and throat (ENT) department of the hospital of Wiener Neustadt. The patient reported a massive sore throat and a slightly elevated temperature for four days (See Fig. 1). Clinical examination showed kissing tonsils covered with membranes with the left tonsil partly destroyed. Flexible endoscopy (See Fig. 2) revealed purulent secretion and thick membranes on the whole pharyngeal wall. The neck lymph nodes were bilaterally swollen. An empiric intravenous (i.v.) antibiotic therapy with amoxicillin/clavulanate (2.2 g every 8 h) was started. In addition, 500 mg prednisolone i.v. were administered combined with adrenaline inhalations and analgetic therapy. A peritonsillar abscess was ruled out by computed tomography (CT). An infectious diseases expert was consulted by telephone. The case was described in detail, and the continuation of amoxicillin/clavulanate was recommended.
timeline of disease progression. ENT ear nose and throat, CT computed tomography, ICU intensive care unit, IU international units, C. Corynebacterium, PCR polymerase chain reaction, VV ECMO veno-venous extracorporeal membrane oxygenation, CVVHDF continuous veno-venous hemodiafiltration, VA ECMO veno-arterial extracorporeal membrane oxygenation
Because of increasing constriction of the airway, intubation had to be performed the next day. Over the next hours oxygenation worsened and resulted in acute respiratory distress syndrome (ARDS). On day 6 after symptom onset, the calculated antibiotic therapy was escalated to meropenem (1 g i.v. every 8 h) and linezolid (600 mg every 12 h) because of markedly rising inflammation markers (see Table 1). Vasopressors had to be started due to hemodynamic instability. Acute kidney failure developed. Due to the rapid onset of multi-organ failure and persistent suspicion of diphtheria, a different infectious diseases department was contacted.
Subsequently, the patient was airlifted to Clinic Favoriten, which is the only treatment center in Austria storing diphtheria antitoxin. There, 100.000 I.U. diphtheria-antitoxin were administered i.v. following the instructions of the manufacturer. In the consecutive bronchoscopy the full extent of the membranes, reaching all the way to the lung periphery, became visible. Inflammation markers increased further, the platelet count decreased, and acute kidney injury persisted. The antibiotic therapy was escalated with additional azithromycin (500 mg i.v. once daily) and caspofungin. Due to severe ARDS, the patient was turned into prone position. Catecholamine demand was increasing, and the patient showed bleeding stigmata in the mouth and nose.
On day 7 after symptom onset C. diphtheriae was confirmed in the throat swab and toxin-gene polymerase chain reaction (PCR) was positive for subunits A and B. On the same day the patient desaturated down to 85% saturation of peripheral oxygen (SpO2). After ruling out all reversible causes for hypoxemia an ECMO center was contacted for the implantation of a veno-venous extracorporeal membrane oxygenation (VV ECMO (1)). Because of the patient’s respiratory instability, the implantation was performed by an ECMO retrieval team on site.
After the oxygenation had been restored, the patient was referred to a specialized ECMO-ICU. There, he presented with hyperdynamic septic shock, ARDS, acute kidney failure, and disseminated intravascular coagulation (DIC). Shock management consisted of high-frequent dynamic assessment of fluid responsiveness, large volumes of crystalloid and albumin infusions, high-dose combination vasopressor therapy including norepinephrine, epinephrine, and argipressin, as well as hydrocortisone i.v. Continuous veno-venous hemodiafiltration was started and, in the context of diffuse mucosal bleeding, DIC management required the administration of clotting factors and thrombocyte concentrates. Accordingly, ECMO was administered without any anticoagulation therapy throughout the course. On day 8, the patient developed additional ischemic hepatitis and, ultimately, septic cardiomyopathy with resulting hypodynamic septic shock. Thus, VV ECMO had to be switched to venoarterial ECMO [5] after a trial of dobutamine had failed. On day 9, the patient presented with wide, light rigid pupils. A cerebral CT scan revealed intracerebral spot bleedings and cerebral swelling. The young man died 9 days after the symptom onset of respiratory diphtheria.
Microbiology and molecular investigation
Both pharyngeal samples yielded growth of Corynebacterium diphtheriae var mitis. PCR for diphtheria toxin subunits A and B [6] was positive, and toxin production was confirmed by ELEK-test. Minimum inhibitory concentrations (MICs) of antimicrobials were determined by Etest (bioMerieux SA, Marcy l’Etoile, France), in accordance with the manufacturer’s instructions. The MICs of ten antimicrobials and where available, interpretations according to EUCAST version 12.0 [7] and CLSI M45 2015 guideline [8], are shown in Table 2. One of the pharyngeal isolates was sequenced with Next Seq 2000 (Illumina, San Diego, CA, USA), with 151 bp paired-end reads. C. diphtheriae sequences were de novo assembled using SPAdes algorithm and further analyzed with Seqsphere + client version 8.3.4 (Ridom, Muenster, Germany). A core genome multilocus sequence type (cgMLST) comprising 1494 targets an accessory genome comprising 660 targets were created with one seed genome and 35 query genomes, thereof 13 complete genomes (bold), 1 chromosome, and 21 whole genome shotgun sequencing projects. (NCBI accession numbers: reference genome: *NC_002935.2, query Genomes (35): *NC_016782.1, *NC_016799.1, *NC_016800.1, *NC_016801.1, *NC_016785.1 *NC_016786.1, *NC_016802.1, *NC_016787.1, *NC_016788.1, *NC_016783.1, *NC_016789.1, *NC_016790.1, *NZ_CP018331.1, **NZ_LN831026.1 chromosome 1, MIOA00000000.1, *MINY00000000.1, *MINZ00000000.1, *MIOB00000000.1, *MIYN00000000.1, *MIOG00000000.1, *MIOJ00000000.1, *MIOK00000000.1, *MIOC00000000.1, *MIOD00000000.1, *MIOE00000000.1, *MIOL00000000.1, *MIOM00000000.1, *MIYO00000000.1, *MIYQ00000000.1, *MION00000000.1, *MIYS00000000.1, *MIYP00000000.1, *MIOO00000000.1, *MIOP00000000.1 and *MIOR00000000.1.).
The isolate was assigned to sequence type (ST) 574 with the MLST profile atpA: 2, dnaE: 10, danK: 3, fusA: 1, leuA: 3, odhA: 3, rpoB: 2 and was confirmed to carry the diphtheria toxin gene.
Infection control
The case of respiratory diphtheria was reported to the Austrian health authority, which started contact tracing: the patient had first been registered in Austria on 20th May 2022, the day of symptom onset. He had shared a room with three other asylum seekers, who were all untraceable.
Discussion
In Austria, no infections with C. diphtheriae have been detected from 1993 until 2014, when a case of cutaneous diphtheria was diagnosed in a teenager from Eastern Africa [4]. Since then, occasional infections or colonization with toxigenic C. diphtheriae and ulcerans have been reported. Up to the present case, none of these infections have shown a toxic disease course [9].
In Europe, only rare cases of respiratory diphtheria have been recently reported: A 6-year old in Spain survived the infection in 2015 [10]. Respiratory diphtheria was lethal for an unvaccinated child in Belgium in 2016 [11]. In 2018 two cases have been reported in Spain and Latvia [12].
In Afghanistan a diphtheria outbreak was reported in 2002 with 854 reported cases (40.7 incidence rate (IR)). Subsequently, the incidence had declined down to zero reported cases. Since 2018 cases have been rising again to 61 in the year 2020 (1.5 IR) [13]. Globally, the diphtheria incidence is currently declining with 8638 reported cases in 2021 (1.3 IR) after a peak of 22,986 cases in the year 2019 (3.4 IR). Most of these cases in 2019 have been reported in the WHO African region (17.4 IR) followed by the WHO South-East Asian region (5.1 IR) [13]. These data include respiratory and cutaneous diphtheria [14].
The administration of diphtheria antitoxin is crucial for the successful treatment of respiratory diphtheria [15]. It must be given as soon as possible, ideally at once when diphtheria is suspected [16], and must not be delayed to wait for laboratory confirmation [16]. The antitoxin consists of neutralizing antibodies that can only bind the diphtheria toxin before cell entry [17]. In the cell, the toxin irreversibly causes cell death by inactivating elongation factor 2 [18]. Antibiotics have no effect on the circulating diphtheria toxin and are therefore insufficient as treatment [16], but they can inhibit the production of more diphtheria toxin, and they effectively prevent disease transmission [16]. The initial empirical antibiotic therapy with amoxicillin clavulanate in the present case was chosen because the infectious diseases expert mainly suspected bacterial tonsillitis in the patient’s very early disease course with the differential diagnoses of angina Plaut Vincenti or Lemierre’s syndrome.
Various antibiotics can be used for diphtheria treatment including penicillin, erythromycin and other macrolide antibiotics, clindamycin as well as linezolid and vancomycin in resistant strains. The bactericidal penicillin or the bacteriostatic erythromycin are recommended as first line therapy [19]. A small study comparing penicillin and erythromycin found a shorter time to fever clearance in the penicillin group, but also one patient with treatment failure and one with relapse. 27% of the isolates were resistant to erythromycin [20].
In 2020, 12 strains of C. diphtheriae and ulcerans have been detected in Austria from human and animal samples. None of the samples caused respiratory infections. Using the breakpoints of the European Committee of Antimicrobial Susceptibility testing [7], all isolates were sensible for linezolid and rifampicin. Three isolates (23%) were resistant to clindamycin, five (38%) were resistant to ciprofloxacin and five (38%) to penicillin [21].
The authors found no data supporting the use of protein synthesis inhibiting antibiotics such as linezolid and clindamycin in diphtheria. No evidence exists for combined antibiotic treatment. Because of reports of antimicrobial resistances to erythromycin [20, 22, 23], penicillin [23, 24], clindamycin [25] and multidrug resistance in 10.4% [23], combined antibiotic therapy is probably reasonable until antimicrobial resistance testing has been performed. Clindamycin resistance is especially frequent in C. ulcerans [26]. The broad antibiotic therapy in the present case was chosen to empirically cover also other causes of ARDS and multiorgan failure in the context of a life-threatening infection.
The team was notified about the positive result of the diphtheria toxin PCR four days after the first hospital contact, when the swab was taken. This delay was partly caused by a public holiday in Austria when microbiological laboratories are not working. Furthermore, the sample had to be transported 60 km from the rural hospital in Wiener Neustadt to the diphtheria reference center in Vienna where the toxin PCR is available.
In the present case, antitoxin was administered at a progressed stage of the disease course. This delay might be explained by a lack of knowledge on antitoxin availability as well as the loss of familiarity with this rare disease. Information about antitoxin availability is therefore crucial and must be shared with health care providers to prevent any delay of antitoxin administration in unvaccinated patients with respiratory diphtheria [27]. Immuno-absorption therapy was not used because of a lack of scientific evidence for immune-absorption therapy. Furthermore, a positive effect was not expected by the treating physicians because the circulating antitoxin was neutralized by the antitoxin, and the intracellular toxin was not available for immuno-absorption.
The diphtheria toxoid vaccination protects with an efficacy of 98% against toxic diphtheria disease [2]. Poor health care systems, vaccine skepticism, religious beliefs, fundamentalism, and lack of awareness because of the rarity of the disease might all be factors leading to insufficient vaccine coverage in low [28] and highly developed [29, 30] countries. The seroprevalence of diphtheria antibodies is decreasing with age because of low booster vaccination rates [29, 30]. Diphtheria toxoid vaccine coverage of children in Austria used to be about 90%. Disturbingly, a decrease down to 85% full vaccine coverage has been reported since 2018 [31].
A study, that was carried out in 2013 in Afghanistan, found that 31% of children were only partially vaccinated according to the Afghan immunization plan, and 18% were not vaccinated at all. The vaccine coverage rate ranged grossly between Afghan provinces from 2.5% to 83% [32]. After 2013 immunization programs have been reinforced, improving the diphtheria toxoid vaccine coverage in children to a peak of 72% in 2018/2019 [33]. Since then, vaccine coverage in children has been declining, presumably because of political instability and the Taliban’s seizure of power.
Due to insufficient vaccination coverage and ongoing strong migration movements, further cases of respiratory diphtheria must be expected in Austria and other European countries. Awareness of the importance of early antitoxin administration is the key to prevent further lethal cases.
Data availability
More data is available by contacting the corresponding author.
Change history
13 January 2023
A Correction to this paper has been published: https://doi.org/10.1007/s15010-022-01964-y
References
Behring E, Kitasato S. Ueber das Zustandekommen der Diphtherie-Immunität und der Tetanus-Immunität bei Thieren. Deutsche Medizinische Wochenschrift. 1890;49.
Acosta AM, Moro PL, Hariri S, Tiwari TSP. Epidemiology and prevention of vaccine-preventable diseases. The Pink Book: Diphtheria. Centre of Disease Control and Prevention: CDC Pink Book; 2021.
WHO. Diphtheria reported cases and incidence, European Region; 2022. https://immunizationdata.who.int/pages/incidence/DIPHTHERIA.html?CODE=EUR&YEAR=.
Huhulescu S, Hirk S, Zeinzinger V, Hasenberger P, Skvara H, Müllegger R, et al. Letter to the editor: cutaneous diphtheria in a migrant from an endemic country in east Africa, Austria May 2014. Eurosurveillance. 2014;19(26):20845.
Conrad SA, Broman LM, Taccone FS, Lorusso R, Malfertheiner MV, Pappalardo F, et al. The extracorporeal life support organization maastricht treaty for nomenclature in extracorporeal life support. A position paper of the extracorporeal life support organization. Am J Respir Crit Care Med. 2018;198:447–51.
Mothershed EA, Cassiday PK, Pierson K, Mayer LW, Popovic T. Development of a real-time fluorescence PCR assay for rapid detection of the diphtheria toxin gene. J Clin Microbiol. 2002;40:4713–9.
The European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters. Version 12.0. 2022. http://www.eucast.org.
CLSI. Methods for antimicrobial dilution and disk susceptibility testing of infrequently isolated or fastidious bacteria. 3rd ed. CLSI guideline M45. Wayne, PA: Clinical and Laboratory Standards Institute; 2015. https://clsi.org/standards/products/microbiology/documents/m45/.
Bundesministerium für Soziales, Gesundheit, Pflege und Konsumentenschutz (BMSGPK). Nationale Referenzzentrale für Diphtherie-Labor Jahresbericht 2021. Vienna 2022. https://www.sozialministerium.at.
European Centre for Disease Prevention and Control. A case of diphtheria in Spain, 15 June 2015. Stockholm: ECDC; 2015.
European Centre for Disease Prevention and Control. A fatal case of diphtheria in Belgium, 24 March 2016. Stockholm: ECDC; 2016.
European Centre for Disease Prevention and Control. Diphtheria. In: ECDC. Annual epidemiological report for 2018. Stockholm: ECDC; 2021.
Diphtheria reported cases and incidence [Internet]. World Health Organization; 2022. https://immunizationdata.who.int/pages/incidence/DIPHTHERIA.html?CODE=AFG&DISEASE=DIPHTHERIA.
WHO. Diphtheria, Vaccine preventable diseases, Surveillance standards. 2018.
Kadirova R, Kartoglu HU, Strebel PM. Clinical characteristics and management of 676 hospitalized diphtheria cases, Kyrgyz Republic, 1995. J Infect Dis. 2000;181:S110–5.
Begg N, World Health Organization, Regional Office for Europe. Manual for the management and control of diphtheria in the European region. Copenhagen: WHO Regional Office for Europe; 1994. https://apps.who.int/iris/handle/10665/108107.
MacGregor RR. In: Principles and Practice of Infectious Diseases te, Churchill Livingstone, Philadelphia 2005. Corynebacterium diphtheriae. In: Mandell G, Bennett JRD, editors. Principles and Practice of Infectious Diseases. Philadelphia: Churchill Livingstone; 2005. p. 2457.
Choe S, Bennett MJ, Fujii G, Curmi PMG, Kantardjieff KA, Collier RJ, et al. The crystal structure of diphtheria toxin. Nature. 1992;357:216–22.
RKI. Diphtherie RKI-Ratgeber. In: Institut RK, editor. Merkblaetter. rki.de; 2018.
Kneen R, Pham NG, Solomon T, Tran TM, Nguyen TT, Tran BL, et al. Penicillin vs. erythromycin in the treatment of diphtheria. Clin Infect Dis. 1998;27(4):845–50.
Bundesministerium für Soziales G, Pflege und Konsumentenschutz (BMSGPK). Jahresbericht Diphtherie 2020. 2020.
Engler KH, Warner M, George RC. In vitro activity of ketolides HMR 3004 and HMR 3647 and seven other antimicrobial agents against Corynebacterium diphtheriae. J Antimicrob Chemother. 2001;47(1):27–31.
Hennart M, Panunzi LG, Rodrigues C, Gaday Q, Baines SL, Barros-Pinkelnig M, et al. Population genomics and antimicrobial resistance in Corynebacterium diphtheriae. Genome Med. 2020;12(1):107.
Forde BM, Henderson A, Playford EG, Looke D, Henderson BC, Watson C, et al. Fatal respiratory diphtheria caused by ß-lactam–resistant Corynebacterium diphtheriae. Clin Infect Dis. 2020;73(11):e4531–e8.
Coyle MB, Minshew BH, Bland JA, Hsu PC. Erythromycin and clindamycin resistance in Corynebacterium diphtheriae from skin lesions. Antimicrob Agents Chemother. 1979;16(4):525–7.
Moore LSP, Leslie A, Meltzer M, Sandison A, Efstratiou A, Sriskandan S. Corynebacterium ulcerans cutaneous diphtheria. Lancet Infect Dis. 2015;15(9):1100–7.
Both L, White J, Mandal S, Efstratiou A. Access to diphtheria antitoxin for therapy and diagnostics. Euro Surveill. 2014. https://doi.org/10.2807/1560-7917.ES2014.19.24.20830.
Centers for Disease Control and Prevention. Epidemiology and Prevention of Vaccine-Preventable Diseases. Hall E., Wodi A.P., Hamborsky J., et al., eds. 14th ed. Washington, D.C. Public Health Foundation, 2021. https://www.cdc.gov/vaccines/pubs/pinkbook/dip.html
United Nations Children’s Fund. (2019, July 12). 20 million children miss out on lifesaving measles, diphtheria and tetanus vaccines in 2018 [press release]. New York/ Geneva: UNICEF2019. https://www.unicef.org/eca/press-releases/20-million-children-miss-out-lifesaving-measles-diphtheria-and-tetanus-vaccines-2018
Maple PA, Efstratiou A, George RC, Andrews NJ, Sesardic D. Diphtheria immunity in UK blood donors. The Lancet. 1995;345:963–5.
McQuillan GM, Kruszon-Moran D, Deforest A, Chu SY, Wharton M. Serologic immunity to diphtheria and tetanus in the United States. Ann Intern Med. 2002;136(9):660–6.
Mugali RR, Mansoor F, Parwiz S, Ahmad F, Safi N, Higgins-Steele A, et al. Improving immunization in Afghanistan: results from a cross-sectional community-based survey to assess routine immunization coverage. BMC Public Health. 2017;17(1):290.
Immunization, DPT (% of children aged 12-23 months) - Afghanistan: The World Bank; 2020 [Available from: https://data.worldbank.org/indicator/SH.IMM.IDPT?end=2020&locations=AF&start=1980&view=chart
Acknowledgments
The authors would like to thank all additional people involved in the patient care at Krankenhaus Wiener Neustadt, Klinik Favoriten and the 13i2 intensive care unit of the General Hospital of Vienna as well as people involved in the laboratory work at the Austrian Agency of Health and Food Safety (Silke Stadlbauer, Petra Hasenberger and Ernst Amtmann) and the Reference Laboratory for vaccine preventable diseases, London, United Kingdom for performing confirmatory tests.
Funding
No funding was received for this case report.
Author information
Authors and Affiliations
Contributions
MT, BE, TS, AM, JR, SI, JF, MK, MS, TO, WH, SN, and CW: clinically managed the case of respiratory diphtheria in the different departments. SP, FH, AI, and SS: undertook the microbiological testing. MB performed the sequencing and bioinformatic analysis. MT, SP; MB, BE, AM, JR, SI and JF: wrote the first draft of the manuscript. MT, WH, SN and CW: revised the manuscript. All authors reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have any competing interests to declare.
Ethical approval
Because of the fulminant and lethal disease course no informed consent could be obtained from the patient. The Austrian immigration authority did not have any contact details from the family or friends, who could neither be informed about the man’s death nor be asked for permission to write a case report. By raising awareness of diphtheria, this case report can help to prevent further cases of respiratory diphtheria with late antitoxin administration.
Consent to participate
Because of the fulminant and lethal disease course no informed consent could be obtained from the patient. The Austrian immigration authority did not have any contact details from the family or friends, who could neither be informed about the man’s death nor be asked for permission to write a case report. The case report does not contain any pictures that could identify the patient. The patient’s identity is fully anonymized.
Consent for publication
Because of the fulminant and lethal disease course no consent to publish could be obtained from the patient. The Austrian immigration authority did not have any contact details from the family or friends, who could neither be informed about the man’s death nor be asked for permission to write a case report. The case report does not contain any pictures that could identify the patient. The patient’s identity is fully anonymized.
Additional information
The original online version of this article was revised: Fig. 1 in the original version of this article has been replaced.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Traugott, M.T., Pleininger, S., Inschlag-Tisch, S. et al. A case of fulminant respiratory diphtheria in a 24-year-old Afghan refugee in Austria in May 2022: a case report. Infection 51, 489–495 (2023). https://doi.org/10.1007/s15010-022-01926-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-022-01926-4