Abstract
Purpose
Echinococcus multilocularis infects humans as a false intermediate host, primarily with intrahepatic manifestation. Incorrect diagnostic interpretation of these liver tumors, especially the hemangioma-like pattern, can lead to progressive disease. The aim of the study was to investigate the differentiation of typical hemangioma and a hemangioma-like pattern of E. multilocularis using contrast-enhanced ultrasound (CEUS).
Methods
This prospective clinical pilot study comprised patients with hemangioma (n = 14) and patients with alveolar echinococcosis (AE) and hemangioma-like pattern (n = 7). Inclusion criteria were the detection of a liver lesion according to a hemangioma-like pattern on E. multilocularis Ulm classification—ultrasound (EMUC-US) and “confirmed” or “probable” AE according to WHO case definition. The comparison group had hepatic hemangioma with typical B-scan sonographic morphology. All participants underwent conventional and contrast-enhanced ultrasonography.
Results
The patient group comprised five men (71.4%) and two women (28.6%) with a mean average age of 64.1 ± 11.2 years. The patient group with hemangioma comprised nine female subjects (64.3%) and five male subjects (35.7%) with a mean average age of 56.1 ± 12.0 years. Early arterial bulbous ring enhancement (p < 0.0001) and iris diaphragm phenomenon could only be visualized in the patients with hemangioma (p < 0.0001). Furthermore, the patients with hemangioma exhibited hyperenhancement in the late phase (p = 0.0003). In contrast, the patients exhibited typical early arterial rim enhancement (p < 0.0001) and, in the portal venous and late phase, complete or incomplete non-enhancement (black hole sign; p = 0.0004).
Conslusion
The behavior of hemangioma-like AE lesions and typical liver hemangiomas is significantly different on CEUS. AE should be considered as a possible differential diagnosis, especially in high-endemic areas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fox tapeworm disease, alveolar echinococcosis (AE) is a zoonosis caused by the larval stage of the cestode Echinococcus multilocularis, for which humans may act as a false intermediate host. The worm eggs are ingested accidentally, penetrate the small intestinal wall and travel to organs via the blood or lymphatic vessels. In a majority of cases, AE primarily affects the liver and forms infiltrative alveolar space-occupying lesions, similar to a malignant tumor [1, 2].
Increasing prevalence and incidence, and spread beyond the classic endemic areas, have been reported worldwide [3]. After infection with E. multilocularis, fox tapeworm disease is characterized by an asymptomatic progressive course. Five to 15 years can pass before a diagnosis of AE is made [1, 2]. Due to the potentially lethal course of AE, therapy must be initiated after diagnosis [1]. First-choice therapy is radical surgical resection [4]. In the case of inoperability, life-long oral antihelmintic therapy with benzimidazole (BZM) is usually required [5]. However, initial data indicate that negative serology for echinococcus and lack of [18F]FDG uptake in 18FDG positron emission tomography (PET) suggests that discontinuation of mebendazole therapy is feasible [6,7,8].
The diagnosis of AE is based on the combination of imaging, clinical and serological findings [4]. As an inexpensive, radiation-free and readily available technique, ultrasound is the method of choice for detecting the disease [9]. The heterogeneous sonomorphology of hepatic AE, especially the metastasis- and hemangioma-like pattern, poses a major challenge in clinical practice [10].
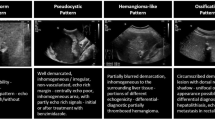
The E. multilocularis Ulm classification—ultrasound (EMUC-US) was the first to systematically describe the different morphologies [10] (Fig. 1). A total of five following different patterns are distinguished: hailstorm, pseudocystic, hemangioma-like, metastasis-like and ossification [10]. CT and MRI are additional methods that can be used in the diagnosis of hepatic AE [11,12,13]. However, PET-CT and PET-MRI are the only methods used to assess the parasitic activity of AE lesions [14, 15]. More than 30% of patients are misdiagnosed during the diagnostic workup [16]. This can lead to delayed therapy, progression of the disease and an increase in complication-rich and inoperable disease courses [5, 16]. Differentiation of typical hemangiomas and a hemangioma-like pattern according to EMUC-US is not possible with B-scan ultrasonography alone.
Echinococcus multilocularis Ulm classification—ultrasound (EMUC-US) [10]
Contrast-enhanced ultrasound (CEUS) has emerged in recent years as another diagnostic technique in AE. However, the studies that have investigated the use of CEUS in hepatic AE have been almost exclusively retrospective, in rodents, or not used a classification system [11, 17,18,19,20,21]. No prospective studies investigating the hemangioma-like pattern in AE are available. Hemangiomas are one of the most common tumors in the liver, along with cysts [22]. Contrast-enhanced sonography is the method of choice in the workup of typical hemangiomas [23, 24].
Based on the knowledge that the use of contrast-enhanced sonography already allows more accurate characterization of AE lesions, this prospective study is the first to investigate the differences in the contrast response of patients with hemangioma-like pattern classified by EMUC-US and subjects with typical liver hemangiomas. The aim of this study was to investigate the extent to which “typical true hemangiomas” indistinguishable sonomorphologically on B-scan differ from hemangioma-like lesions in hepatic AE on CEUS.
Methods
Ethics statement
This study was approved by the local ethics committee and conducted in accordance with the Declaration of Helsinki (ref. no. 17/21 and 23/20). All data were analyzed pseudo-anonymously.
Study collective
The prospective clinical study was conducted from March 2021 to June 2021 (Fig. 2). The study population consisted of a patient group with hemangioma-like pattern in alveolar echinococcosis and a comparison group with typical hemangioma. Patients with AE were recruited from the National Echinococcosis Registry [25]. Patients were contacted by telephone or mail as part of the prospective study and were invited to participate in the study. Inclusion criteria were the presence of a liver lesion with hemangioma-like pattern classified by EMUC-US and confirmed or probable AE disease according to WHO case definition “confirmed” or “probable” [4] (Fig. 3a). For the comparison group, patients with simple liver hemangioma on central ultrasound were recruited during the same time period. During the study period, 15 consecutive patients who presented for evaluation of hemangiomas in our ultrasound department were recruited and asked to participate in the study. Inclusion in the patient group with hemangioma required liver hemangioma with typical B-scan sonographic morphology (round to oval shape, homogeneously echo-rich, sharp border) (Fig. 3b).
Medical history
All patients were asked about their current health status before enrollment in the study and were appropriately examined during sonography if they had symptoms. A comprehensive vegetative and anthropometric history (age, height, weight) was obtained from all patients and subjects before conventional ultrasound and CEUS were performed.
Ultrasound examinations
Ultrasound examinations were performed using Canon/Toshiba Aplio i800 (convex probe, C6-1MHz) and Toshiba Aplio 500 (convex probe, C5-2MHz; Toshiba i8CX1, Canon Medical Systems Corporation) ultrasound scanners. In the B-scan, the liver was scanned in tissue harmonic imaging (THI) mode, and all fox tapeworm lesions and hemangiomas were measured at their greatest extent in at least two planes, assigned to a liver segment (according to Couinaud), precisely described morphologically and examined for blood flow using standard Doppler techniques (color-coded/power (CPA)/advanced dynamic imaging (ADF)/superb microvascular imaging (SMI) Doppler). If there was evidence of cholestasis or tenderness, this was also noted on the examination form. The lesion with the largest diameter was the reference lesion.
Contrast-enhanced ultrasound
Following the screening of the liver in the B-scan, CEUS was performed according to the “Guidelines and Good Clinical Practice Recommendations for Contrast Enhanced Ultrasound in the Liver—Update 2020” of the World Federation for Ultrasound in Medicine and Biology [26]. For a better overview of the liver parenchyma, twin mode was activated and the device standardized to 8.00–22.00 frames per second (fps), a low mechanical index (MI) of 0.06–0.09, a gain of 84.00 on average (range 71.00–88.00) and a dynamic range of 60.00–75.00. After selecting an optimal transducer position for the examination with the best possible visualization of the reference lesion, 1.2–1.8 ml of SonoVue® (Bracco Medical Imaging Deutschland GmbH, Konstanz, Germany) was administered intravenously via an indwelling vein cannula within 2 s, followed by 10 ml of 0.9% NaCl solution. Two subjects received 1.2–1.8 ml SonoVue® again for better visualization of the reference lesion. “Contrast arrival time” was defined as the time until the contrast agent flared up, marking the beginning of the arterial phase, which changes to the portal venous phase after 30 s due to increasing uptake of contrast medium by the liver parenchyma. The portal venous phase is followed by the late phase after 120 s. The reference lesion was observed by CEUS for a total of 4 min. Compared to the surrounding liver parenchyma, the contrast agent behavior of the reference lesion was semi-quantitatively described as hyper-, iso-, hypo-, or non-enhancement in the arterial, portal venous and late phases by an experienced ultrasound investigator. In hepatic hemangiomas, typical behavior on CEUS has been defined as early arterial bulbous ring enhancement followed by complete or incomplete iris diaphragm phenomenon [23, 27].
Statistical analysis
Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). First, the frequencies, means, medians and location and dispersion measures were calculated. Normal distribution was assessed using the Shapiro–Wilk test. For a comparison of interval-scaled variables, the non-parametric Mann–Whitney U test was used. Pearson’s χ2 test and Fisher’s exact test were used to determine possible relationships and differences in frequency distributions between dichotomous variables. p-values < 0.05 (α = 0.05) were considered significant with a 5% probability of error.
Results
Study collective
The patient group (n = 7) included five men (71.4%) and two women (28.6%). At the time of the study, the mean average age was 64.1 ± 11.2 years and the mean BMI was 24.9 ± 3.9 kg/m2. Five patients (71.4%) were classified as “probable” and two (28.6%) as “confirmed” according to the WHO case definition for AE. The mean duration of disease in AE patients was 47.3 ± 28.5 months. At diagnosis, three patients (42.9%) reported symptoms, including upper abdominal tenderness and fullness. Four patients (57.1%) were asymptomatic and the diagnosis of AE was made as an incidental finding. All patients were treated with oral antihelmintic therapy. The patient group with hemangioma (n = 14) included nine female subjects (64.3%) and five male subjects (35.7%). The mean average age was 56.1 ± 12.0 years and the mean BMI was 25.1 ± 2.8 kg/m2. There were no significant differences in gender, age, or BMI between the patients with hemangioma and patient with hemangioma-like pattern in AE (Table 1).
Lesion size, localization and morphology on the B-scan
A total of 12 AE lesions and 17 hemangiomas were detected on B-scan. Four patients with AE (57.1%) and 12 patients with hemangioma (85.7%) presented with one solitary liver lesion, one patient with AE (14.3%) and one patient with hemangioma (7.1%) with two, and two patients with AE (28.6%) and one patient with hemangioma (7.1%, 1/14) with three hepatic space-occupying lesions. The mean lesion size was 61.9 ± 30.0 mm for the AE patients and 21.2 ± 12.8 mm in patients with hemangioma. The difference in the mean reference lesion size between the patients with hemangioma and patients with AE was significant (p = 0.0019).
The reference lesion was located left hepatic in two patients with AE (28.6%) and five patients with hemangioma (35.7%) and right hepatic in two patients with AE (28.6%) and nine with hemangioma (64.3%). Bihepatic involvement was observed in three patients (42.9%).
Significant differences were found in the morphology of the liver lesions in regard to the shape of the space-occupying lesions; 6 (85.7%) of the AE lesions had a polycyclic shape, whereas all 14 (100.0%) hemangiomas had round-oval presentation (p = 0.00012). Furthermore, we found significant differences in the lesions in terms of demarcation from the liver parenchyma; all 7 (100.0%) AE lesions had blurred demarcation, whereas 11 (78.6%) hemangiomas were sharply demarcated from the liver tissue and the remaining 3 (21.4%) had slightly blurred demarcation (p < 0.0001). Regarding homogeneity, significant differences were identified between the space-occupying lesions (100.0%) inhomogeneous presentation of AE lesions vs. 85.7% [12/14] homogeneous hemangiomas and 14.3% [2/14] inhomogeneous hemangiomas (p = 0.00031). All hemangiomas presented without perfusion using standard Doppler techniques (Table 2).
Behavior of lesions in CEUS
A mean contrast arrival time of 14.7 ± 4.9 s was determined for patients and 11.6 ± 2.1 s for patients with hemangioma.
AE lesions
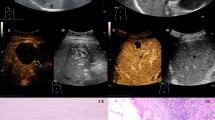
All AE lesions presented early arterial rim enhancement in the arterial phase (p < 0.0001) (Fig. 4a). In one patient (14.3%), this presented as atypical and fan-shaped. An iris phenomenon was not observed in any AE lesion during the portal venous phase (p < 0.0001) (Fig. 5a). All AE lesions presented with central non-enhancement (complete or incomplete) in the portal venous and late phases (p = 0.0004) (Fig. 6a). No significant difference was found for the irregular central hypo-enhanced internal echoes observed in two (28.6%) AE patients (p = 0.1000) (Table 2).
a In the late phase, the AE lesion continues to show no contrast uptake. Further demonstration of the existing black hole phenomenon; b complete contrast uptake in the sense of a complete iris diaphragm phenomenon and echo-rich visualization of the hemangioma compared to the adjacent liver parenchyma
Hemangiomas
In contrast, bulbous ring enhancement was observed in all hemangiomas in the arterial phase (p < 0.0001) (Fig. 4b). In the portal venous phase, 3 hemangiomas (21.4%) presented incomplete and 11 (78.6%) complete iris phenomenon (p < 0.0001) (Fig. 5b). Of the 14 hemangiomas, 12 presented with hyper-enhancement (p = 0.0003) and 2 with iso-enhancement (p = 0.5333) in the late phase (Fig. 6b, Table 2).
Discussion
This is the first prospective clinical study to evaluate AE patients with a hemangioma-like pattern classified by EMUC-US in contrast-enhanced sonography and compare them to patients with typical hepatic hemangioma. The results of this study support the hypothesis that typical hemangiomas and AE space-occupying hemangioma-like lesions differ significantly on CEUS.
Multiple prior studies also observed early arterial rim enhancement and black hole sign in fox tapeworm lesions [11, 17,18,19,20,21]. However, these studies were mostly performed retrospectively or in rodents and did not classify the AE lesions according to EMUC-US.
The hemangioma-like pattern of AE and its difficult differential diagnosis from atypical hemangioma on B-scan ultrasonography was first described by Bresson-Hadni et al. in 2006 [28]. Consistent with our study results, Cai et al. postulated that CEUS, in contrast to B-scan ultrasound, provides better visualization of morphological and vascular structures in the differential diagnosis of AE. In their study, five AE lesions could be diagnosed only by CEUS. On B-scan ultrasonography, the echo-rich space-occupying lesions were misinterpreted as typical hemangiomas [17]. Comparative studies with other diagnostic modalities and liver lesions of other entities are necessary to establish CEUS as a diagnostic tool in AE with atypical sonomorphology. However, such comparative studies are currently lacking. In addition to EMUC-US, classification systems currently exist for MRI and CT [12, 13]. In 2015, Azizi et al. correlated MR tomographic AE lesions classified by Kodama with [18F]FDG uptake on PET-CT and demonstrated increased PET activity for type I–III microcysts [29]. Given the potential for AE to change its morphological presentation during the course of disease, the retrospective design of this investigation with sometimes months-long intervals between diagnostic modalities represents a major shortcoming [30]. Our study results support the need to perform a one-time CEUS to exclude fox tapeworm disease in the case of an incidentally first diagnosed echo-rich mass of the liver (suspected typical hemangioma) in high-endemic areas and the concomitant presence of risk factors for AE [31,32,33].
Limitations
The small number of included patients with AE with a hemangioma-like pattern must be considered a limitation. Furthermore, the patients with hemangioma consisted of nine women and only five men. However, a possible different contrast behavior of hemangiomas between women and men has not been described in the literature so far. Due to the rarity of the disease in Germany, further multicenter studies are necessary to confirm the results.
Conclusions
Our work shows that hemangioma-like AE lesions classified by EMUC-US differ from typical liver hemangiomas on CEUS. In the presence of early arterial rim enhancement and black hole sign, the diagnosis of AE should be considered. When a hemangioma-like liver lesion is first diagnosed, CEUS of the liver should be performed in high-endemic areas to rule out hepatic AE.
Data sharing statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
Ammann RW, Eckert J. Cestodes. Echinococcus. Gastroenterol Clin North Am. 1996;25:655–89.
Kern P, Menezes da Silva A, Akhan O, Müllhaupt B, Vizcaychipi KA, Budke C, Vuitton DA. The Echinococcoses: diagnosis, clinical management and burden of disease. Adv Parasitol. 2017;96:259–369.
Baumann S, Shi R, Liu W, Bao H, Schmidberger J, Kratzer W, Li W, interdisciplinary Echinococcosis Working Group Ulm. Worldwide literature on epidemiology of human alveolar echinococcosis: a systematic review of research published in the twenty-first century. Infection. 2019;47:703–27.
Brunetti E, Kern P, Vuitton DA, Writing Panel for the WHO-IWGE. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop. 2010;114:1–16.
Grüner B, Kern P, Mayer B, Gräter T, Hillenbrand A, Barth TEF, Muche R, Henne-Bruns D, Kratzer W, Kern P. Comprehensive diagnosis and treatment of alveolar echinococcosis: A single-center, long-term observational study of 312 patients in Germany. GMS Infect Dis. 2017;5:Doc01.
Ammann RW, Stumpe KD, Grimm F, Deplazes P, Huber S, Bertogg K, Fischer DR, Müllhaupt B. Outcome after discontinuing long-term benzimidazole treatment in 11 patients with non-resectable alveolar echinococcosis with negative FDG-PET/CT and anti-EmII/3-10 serology. PLoS Negl Trop Dis. 2015;9:e0003964.
Husmann L, Muehlematter UJ, Grimm F, Ledergerber B, Messerli M, Kudura K, Gruenig H, Muellhaupt B, Hasse B, Huellner MW. PET/CT helps to determine treatment duration in patients with resected as well as inoperable alveolar echinococcosis. Parasitol Int. 2021;83:102356.
Reuter S, Buck A, Manfras B, Kratzer W, Seitz HM, Darge K, Reske SN, Kern P. Structured treatment interruption in patients with alveolar echinococcosis. Hepatology. 2004;39:509–17.
Liu W, Delabrousse É, Blagosklonov O, Wang J, Zeng H, Jiang Y, Wang J, Qin Y, Vuitton DA, Wen H. Innovation in hepatic alveolar echinococcosis imaging: best use of old tools, and necessary evaluation of new ones. Parasite. 2014;21:74.
Kratzer W, Gruener B, Kaltenbach TE, Ansari-Bitzenberger S, Kern P, Fuchs M, Mason RA, Barth TF, Haenle MM, Hillenbrand A, Oeztuerk S, Graeter T. Proposal of an ultrasonographic classification for hepatic alveolar echinococcosis: echinococcosis multilocularis Ulm classification-ultrasound. World J Gastroenterol. 2015;21:12392–402.
Li J, Dong J, Yang L, Li X, Song T. Comparison of [18F]Fluorodeoxyglucose positron emission tomography and contrast-enhanced ultrasound for evaluation of hepatic alveolar echinococcosis activity. Ultrasound Med Biol. 2018;44:2199–208.
Graeter T, Schmidberger J. Stage-oriented CT classification and intermodal evolution model in hepatic alveolar echinococcosis. Rofo. 2022;194:532–44.
Kodama Y, Fujita N, Shimizu T, Endo H, Nambu T, Sato N, Todo S, Miyasaka K. Alveolar echinococcosis: MR findings in the liver. Radiology. 2003;228:172–7.
Crouzet J, Grenouillet F, Delabrousse E, Blagosklonov O, Thevenot T, Di Martino V, Piarroux R, Mantion GA, Bresson-Hadni S. Personalized management of patients with inoperable alveolar echinococcosis undergoing treatment with albendazole: usefulness of positron-emission-tomography combined with serological and computed tomography follow-up. Clin Microbiol Infect. 2010;16:788–91.
Lötsch F, Waneck F, Groger M, Auer H, Kaczirek K, Rausch I, Wadsak W, Hacker M, Lagler H, Ramharter M, Karanikas G. FDG-PET/MRI imaging for the management of alveolar echinococcosis: initial clinical experience at a reference centre in Austria. Trop Med Int Health. 2019;24:663–70.
Stojkovic M, Mickan C, Weber TF, Junghanss T. Pitfalls in diagnosis and treatment of alveolar echinococcosis: a sentinel case series. BMJ Open Gastroenterol. 2015;2:e000036.
Cai D, Li Y, Jiang Y, Wang H, Wang X, Song B. The role of contrast-enhanced ultrasound in the diagnosis of hepatic alveolar echinococcosis. Medicine (Baltimore). 2019;98:e14325.
Cai DM, Wang HY, Wang XL, Jiang Y, Luo Y, Li YZ. Ultrasonographic findings of small lesion of hepatic alveolar echinococcosis. Acta Trop. 2017;174:165–70.
Tao S, Qin Z, Hao W, Yongquan L, Lanhui Y, Lei Y. Usefulness of gray-scale contrast-enhanced ultrasonography (SonoVue®) in diagnosing hepatic alveolar echinococcosis. Ultrasound Med Biol. 2011;37:1024–8.
Wa ZC, Du T, Li XF, Xu HQ, Suo-Ang QC, Chen LD, Hu HT, Wang W, Lu MD. Differential diagnosis between hepatic alveolar echinococcosis and intrahepatic cholangiocarcinoma with conventional ultrasound and contrast-enhanced ultrasound. BMC Med Imaging. 2020;20:101.
Zeng H, Wang J, Xie W, Liu W, Wen H. Assessment of early hepatic echinococcus multilocularis infection in rats with real-time contrast-enhanced ultrasonography. Ultrasound Med Biol. 2012;38:1982–8.
Kaltenbach TE, Engler P, Kratzer W, Oeztuerk S, Seufferlein T, Haenle MM, Graeter T. Prevalence of benign focal liver lesions: ultrasound investigation of 45,319 hospital patients. Abdom Radiol (NY). 2016;41:25–32.
Dietrich CF, Sharma M, Gibson RN, Schreiber-Dietrich D, Jenssen C. Fortuitously discovered liver lesions. World J Gastroenterol. 2013;19:3173–88.
Horta G, López M, Dotte A, Cordero J, Chesta C, Castro A, Palavecino P, Poniachik J. Lesiones focales hepáticas benignas: un hallazgo frecuente a la tomografía computada [Benign focal liver lesions detected by computed tomography: Review of 1,184 examinations]. Rev Med Chil. 2015;143:197–202.
Schmidberger J, Kratzer W, Stark K, Grüner B, Echinococcosis Working Group. Alveolar echinococcosis in Germany, 1992–2016. An update based on the newly established national AE database. Infection. 2018;46:197–206.
Dietrich CF, Nolsøe CP, Barr RG, Berzigotti A, Burns PN, Cantisani V, Chammas MC, Chaubal N, Choi BI, Clevert DA, Cui X, Dong Y, D’Onofrio M, Fowlkes JB, Gilja OH, Huang P, Ignee A, Jenssen C, Kono Y, Kudo M, Lassau N, Lee WJ, Lee JY, Liang P, Lim A, Lyshchik A, Meloni MF, Correas JM, Minami Y, Moriyasu F, Nicolau C, Piscaglia F, Saftoiu A, Sidhu PS, Sporea I, Torzilli G, Xie X, Zheng R. Guidelines and good clinical practice recommendations for Contrast-Enhanced Ultrasound (CEUS) in the liver-update 2020 WFUMB in cooperation with EFSUMB, AFSUMB, AIUM, and FLAUS. Ultrasound Med Biol. 2020;46:2579–604.
Strobel D, Seitz K, Blank W, Schuler A, Dietrich CF, von Herbay A, Friedrich-Rust M, Bernatik T. Tumor-specific vascularization pattern of liver metastasis, hepatocellular carcinoma, hemangioma and focal nodular hyperplasia in the differential diagnosis of 1,349 liver lesions in contrast-enhanced ultrasound (CEUS). Ultraschall Med. 2009;30:376–82.
Bresson-Hadni S, Delabrousse E, Blagosklonov O, Bartholomot B, Koch S, Miguet JP, Mantion GA, Vuitton DA. Imaging aspects and non-surgical interventional treatment in human alveolar echinococcosis. Parasitol Int. 2006;55(Suppl):S267–72.
Azizi A, Blagosklonov O, Lounis A, Berthet L, Vuitton DA, Bresson-Hadni S, Delabrousse E. Alveolar echinococcosis: correlation between hepatic MRI findings and FDG-PET/CT metabolic activity. Abdom Imaging. 2015;40:56–63.
Schuhbaur J, Schweizer M, Philipp J, Schmidberger J, Schlingeloff P, Kratzer W. Long-term follow-up of liver alveolar echinococcosis using echinococcosis multilocularis ultrasound classification. World J Gastroenterol. 2021;27:6939–50.
Conraths FJ, Probst C, Possenti A, Boufana B, Saulle R, La Torre G, Busani L, Casulli A. Potential risk factors associated with human alveolar echinococcosis: systematic review and meta-analysis. PLoS Negl Trop Dis. 2017;11:e0005801.
Piarroux M, Piarroux R, Knapp J, Bardonnet K, Dumortier J, Watelet J, Gerard A, Beytout J, Abergel A, Bresson-Hadni S, Gaudart J, FrancEchino Surveillance Network. Populations at risk for alveolar echinococcosis, France. Emerg Infect Dis. 2013;19:721–8.
Kern P, Ammon A, Kron M, Sinn G, Sander S, Petersen LR, Gaus W, Kern P. Risk factors for alveolar echinococcosis in humans. Emerg Infect Dis. 2004;10:2088–93.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was supported by a German Research Foundation (DFG) funded project called “Establishment of a national database for alveolar echinococcosis” (Ref. No. KA 4356/3-1) and “Implementation of interfaces for the standardization of national database systems for alveolar echinococcosis and its transformation processes” (Ref. No. KR 5204/1-2). Further supported by the Ministry of Rural Areas and Consumer Protection Baden-Württemberg “Fuchsbandwurm-Erkrankung: eine Baden-Württembergische Erkrankung (AZ: 14-(33)-8402.43/419E)” and the Bavarian State Government in the context of the funding of the “National Echinococcosis Database Germany” (AZ: K1-2490-PF-2020-FBW) and Müller Holding GmbH & Co. KG Ulm.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by JP, JS, PS and WK. The first draft of the manuscript was written by JP and WK and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
This study was approved by the local ethics committee and conducted in accordance with the Declaration of Helsinki (ref. no. 17/21 and 23/20). All data were analyzed pseudo-anonymously.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publish
Additional informed consent was obtained from all individual participants for whom identifying information is included in this article.
STROBE statement
The authors have read the STROBE Statement checklist of items, and the manuscript was prepared and revised accordingly.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Philipp, J., Schmidberger, J., Schlingeloff, P. et al. Differentiation of hepatic alveolar echinococcosis with a hemangioma-like pattern compared to typical liver hemangioma using contrast-enhanced ultrasound: a pilot study. Infection 51, 159–168 (2023). https://doi.org/10.1007/s15010-022-01866-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-022-01866-z