Abstract
Purpose
Immune function imbalance is closely associated with the occurrence and development of infectious diseases. We studied the characteristics of changes in T-lymphocyte subsets and their risk factors in HIV-negative patients with active tuberculosis (ATB).
Methods
T-lymphocyte subsets in 275 HIV-negative ATB patients were quantitatively analyzed and compared with an Mycobacteriumtuberculosis-free control group. Single-factor and multifactor analyses of clinical and laboratory characteristics of patients were also conducted.
Results
In ATB patients, CD4 and CD8 T-cell counts decreased, and the levels were positively interrelated (r = 0.655, P < 0.0001). After 4 weeks of antituberculosis treatment, CD4 and CD8 T-cell counts increased significantly but remained lower than in the control group. CD4 and CD8 cell counts were negatively associated with the extent of lesions detected in the chest by computed tomography (all P < 0.05). Although not reflected in the CD4/CD8 ratio, CD4 and CD8 cell counts differed between drug-resistant TB patients and drug-susceptible TB patients (P = 0.030). The multivariate analysis showed prealbumin, alpha-1 globulin, body mass index, and platelet count were independent risk factors for decreased CD4 cell count (all P < 0.05), while age and platelet count were independent risk factors for decreased CD8 cell count (all P < 0.05).
Conclusion
CD4 and CD8 T-cell counts showed the evident value in predicting ATB severity. An increase in the CD4/CD8 ratio may be a critical clue of drug resistance in ATB. Although the factors influencing CD4 and CD8 are not identical, our results indicated the importance of serum protein and platelets to ATB patients’ immune function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tuberculosis (TB) is a major infectious disease that seriously threatens human health. One-third of the world’s population is infected with Mycobacterium tuberculosis (Mtb). Among infectious diseases, TB produces the highest single-cause mortality and is a worldwide public health issue that cannot be ignored. The pathogenesis of TB is complex. Compromised immunity is a major risk factor for TB infection [1], while chronic inflammation leads to T-cell dysfunction [2]. The synergistic effect of these factors increases the risk of TB infection and accelerates disease progression.
Attempts to develop alternative novel methods of adjuvant therapy for TB infections have focused on determining the characteristics of the changes in immune function that occur during TB infection and their risk factors. Consensus has emerged that Mtb downregulates the activation of CD4 T-cells through secreted-protein-mediated interference with T-cell proximal and downstream signals, leading to Th1/Th2 imbalance [3] and eventually inducing low expression of CD4 [4, 5]. However, reports on the changes in CD8 T-cells have been inconsistent. Virginie et al. [6, 7] reported an increase in CD8 T-cell numbers, whereas Athman et al. [8, 9] showed a decrease. In terms of risk factors, previous studies have shown that factors including human immunodeficiency virus (HIV), diabetes [10, 11], parasitic infections [12, 13], cavities [14, 15], low 1.25 (OH) 2D3 [16, 17], and high Mtb load [18] can lead to deficiencies in the number and function of T lymphocytes. In addition to these inconsistent results, most existing studies are small-sample, single-factor analyses. Thus, there is an urgent need for in-depth studies in this field.
In this study, we compared the changes in the numbers of CD4 and CD8 T-cells in patients with HIV-negative active tuberculosis (ATB), examined the factors associated with these changes by regression analysis using the optimal scaling method, and further evaluated the degree of correlation. The results provide a foundation for more accurately understanding the changes in immune function in TB patients and providing early intervention in high-risk cases in future clinical practice.
Methods
Study design
We retrospectively analyzed patients with pathogenically positive TB who were continuously hospitalized in Ankang Central Hospital. During the same period, individuals without Mtb infection and clinical symptoms were included as the control. The data were collected between October 1, 2016, and June 30, 2018. A total of 26 variables based on five aspects (the patients’ basic condition, routine examination, protein electrophoresis, bacteriology, and imaging) were collected and analyzed.
Inclusion and exclusion criteria
Inclusion criteria [19]: (1) positive Mtb-deoxyribonucleic acid/ribonucleic acid test accompanied by suspected TB symptoms; (2) one sputum specimen smear-positive for acid-fast staining or culture-positive for Mtb, in addition to chest computed tomography scans that detected lesions consistent with ATB (which might or might not be accompanied by suspected TB symptoms); (3) the patient’s T-lymphocyte subsets had been examined, and the results were available.
Exclusion criteria: (1) individuals who had bilirubin levels ≥ 2 times the upper limit of normal; (2) individuals who were positive for HIV antibody; (3) individuals with nontuberculous mycobacterial lung disease; (4) individuals with concurrent cirrhosis or tumors; and (5) individuals who were taking immunosuppressants.
T-lymphocyte subset assays
T-lymphocyte subset assays were performed using a flow cytometer detection platform (Mindray flow cytometer BriCyte E6, Shenzhen Mindray Bio-Medical Electronics Co., Ltd. Shenzhen, China) and a lymphocyte detection kit (BD Biosciences, Franklin Lakes, NJ, USA). The samples were stored and transported at room temperature (20–25 °C). The samples were assayed within 48 h after collection, and the flow cytometric examination was conducted within 24 h after staining. The main steps were as follows. (1) Fasting venous blood (5 mL) was collected and mixed thoroughly with the anticoagulant EDTA (ethylenediaminetetraacetic acid). (2) The CD3/CD8/CD45/CD4 multitest reagents were added to test tubes (20 μL per tube). (3) Fifty microliters of EDTA-anticoagulated venous blood was added to the bottom of each test tube (the blood sample was not permitted to touch the wall of the test tube). (4) The mixture was thoroughly mixed on a vortexer and incubated for 15 min at room temperature in the dark. (5) After the addition of 450 μL of hemolysin to each test tube, the mixture was mixed gently on a vortex mixer and incubated for 15 min at room temperature in the dark. (6) Automated detection was performed on a flow cytometer.
Measurement of Mtb load and other variables
Mtb load was determined in accordance with the “Diagnostic criteria and principles of management of infectious pulmonary tuberculosis” [19] (Supplementary Table 1) using Roche solid culture medium. The patients were not subjected to repeated analysis. Body mass index (BMI) was calculated as the patient’s body weight (kg) divided by the square of his or her height (m). The patients’ weights, heights, medical histories, and personal histories were collected by the medical staff. The diagnostic criterion for diabetes was random blood glucose ≥ 11.1 mmol/L or fasting blood glucose ≥ 7.0 mmol/L [20]. The extent of the disease was assessed by chest computed tomography (EDCT) in accordance with the standards of the National Tuberculosis Association of the United States [21] (Supplementary Table 2). Whole blood cell counts were performed using a Sysmex XN-9000 automatic blood fluid analyzer and the appropriate reagents. Serum protein electrophoresis was performed on the Sebia Capillarys 2 Flex Piercing platform with the appropriate reagents (Pare Technologique Leonardo da Vinci, CP8010-Lisses 91,008, Evry, CEDEX, France).
Statistical analysis
The normality of the data was assessed using the moment method. The distributions of the data are described by the median and interquartile range (IQR). In univariate analysis, between-group comparisons of dichotomous variables were performed using the Wilcoxon rank-sum test, and between-group comparisons of grades and measurement data were conducted using Spearman correlation analysis. Collinearity was defined as a correlation coefficient greater than 0.7 [22] or a tolerance value lower than 0.1 between two variables before data transformation. The variables that caused collinearity were culled. Missing values were replaced with the series mean. The statistically significant variables in the univariate analysis were subjected to optimal scale regression analysis. Finally, correlation analyses were performed using linear regression equations. All analyses were performed using SPSS 22.0 (IBM Corp., Armonk, NY, USA) and GraphPad Prism 8.0.2 (GraphPad Software, La Jolla, CA, USA) software. P values of less than 0.05 indicated statistically significant differences.
Results
Participant characteristics
A total of 450 individuals were screened for study eligibility, and a total of 331 (73.5%) were included in the study, including 275 confirmed cases of ATB (Fig. 1). The characteristics of the participants are shown in Table 1. In the ATB group, 89.5% (246/275) received the standard nationally recommended chemotherapy regimen. After 4 weeks, 66.2% (182/275) of the patients completed a second test for T-lymphocyte subsets. There was no significant difference in gender or age between the control group and the ATB group (χ2 = 2.280, P = 0.131 and Z = 1761.50, P = 0.689, respectively).
Changes in CD4 and CD8 T-cell numbers
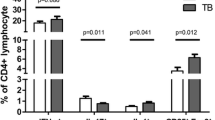
Compared with the control group, the numbers of CD4 T-cells and CD8 T-cells in the ATB group were lower (Z = 850.50, P < 0.0001 and Z = 1504.50, P < 0.0001, respectively; Fig. 2a, b). After 4 weeks of anti-TB treatment, the numbers of CD4 and CD8 T-cells increased markedly in the ATB group compared with the cell numbers before treatment (Z = 37,712.50, P < 0.0001 and Z = 33,919.00, P < 0.0001, respectively; Fig. 2a, b) but were still below the corresponding levels in the control group (Z = 1498.50, P < 0.0001 and Z = 2605.00, P < 0.0001, respectively; Fig. 2a, b). There were no significant differences in the CD4/CD8 ratio among the groups (all P > 0.05; Fig. 2c). In the HIV-negative ATB group, CD4 and CD8 cell numbers were positively correlated with each other (r = 0.655, P < 0.0001; Fig. 2d).
Comparison of CD4 and CD8 T-cell counts at the time of admission with those of patients in the control group and those of patients after anti-TB treatment (a-c). Correlation analysis of CD4 and CD8 T-cell counts in the ATB group (d). The normal distribution values of CD4 and CD8 T-cell counts were converted using Rankit’s formula; CD4 and CD8 T-cell counts were associated with the extent of the disease as assessed by chest computed tomography (e–g). Comparison of CD4/CD8 ratios in patients with drug-susceptible and drug-resistant TB (h). *P < 0.05, ****P < 0.0001. ATB active tuberculosis, BMI body mass index, EDCT extent of the disease as assessed by chest computed tomography, DRTB drug-resistant tuberculosis, DSTB drug-susceptible tuberculosis, TB tuberculosis
CD4 and CD8 are associated with EDCT
Decreases in CD4 and CD8 T-cell counts at the time of admission were negatively correlated with the EDCT results (r = − 0.147, P = 0.015 and r = − 0.127, P = 0.035, respectively; Table 2 and Fig. 2e, f), but the CD4/CD8 ratio showed no correlation with EDCT (r = 0.028, P = 0.647) (Table 2 and Fig. 2g).
Correlation between the CD4/CD8 ratio and drug-resistant TB
Compared with patients with drug-susceptible TB, the number of CD8 T-cells showed a greater decrease in patients with drug-resistant TB, but the difference was not statistically significant (Z = 4057.00, P = 0.442; Table 2). However, it is worth noting that the increase in the CD4/CD8 ratio in the drug-susceptible TB patients was statistically significant (Z = 5378.00, P = 0.030) (Table 2 and Fig. 2h).
Independent risk factors for CD4 and corresponding correlation coefficients
The albumin/globulin ratio was excluded from the multivariate analysis due to collinearity, as its correlation coefficient with albumin was greater than 0.7 (|r| = 0.761). There were no variables with tolerance values less than 0.1 (Supplementary Table 3). The optimal scale regression analysis showed that BMI, platelet count, prealbumin (PA) level, and alpha-1 globulin level were independent risk factors for decreased CD4 T-cell counts (all P < 0.05; Table 3). The correlation analysis showed that CD4 T-cell count was positively correlated with BMI, platelet count, and prealbumin level (r = 0.260, P < 0.0001; r = 0.165, P < 0.006 and r = 0.460, P < 0.0001, respectively; Fig. 3a–c) and negatively correlated with alpha-1 globulin level (r = − 0.285, P < 0.0001; Fig. 3d).
Independent risk factors for CD8 and corresponding correlation coefficients
Age and platelet count were independent risk factors for CD8 T-cell count (all P < 0.05; Table 3). CD8 T-cell count was negatively correlated with age (r = − 0.243, P < 0.0001; Fig. 3e) but positively associated with platelet count (r = 0.163, P = 0.007; Fig. 3f).
Discussion
In this study, through comparing and analyzing T lymphocytes in HIV-negative ATB patients, we characterized changes in CD4 and CD8 T-cell numbers and their relationships with EDCT. We also identified the major factors influencing the numbers of CD4 and CD8 T-cells and the strengths of their correlations.
The results of our study suggest that both CD4 and CD8 T-cell counts are lower in patients with ATB. CD4 T helper cells promote the proliferation and differentiation of immune cells such as CD8 T-cells [1] and B-cells and amplify the immune response, resulting in inhibition of the growth of Mtb. CD4 T-cells play a leading role in the immunity of ATB patients, as shown by many convincing studies worldwide [23] and confirmed by our study, as CD4 and Mtb bacterial load were negatively correlated (r = − 0.122, P = 0.043; Table 2). CD8 T-cells can kill Mtb by particle-mediated effects (such as perforin, granzyme, and others) and can induce cell apoptosis through interaction between Fas and Fas ligands. Our study showed that the number of CD8 T-cells decreased in HIV-negative ATB patients and was positively correlated with CD4 T-cell number, supporting the reduction in CD8 T-cells in HIV-negative ATB and indicating that the reduction in the number of CD4 and CD8 T-cells can be partially reversed by anti-TB treatment. The CD4/CD8 ratio did not reflect the characteristics of the changes in the immune functions of HIV-negative patients with ATB; there was no detectable difference in the CD4/CD8 ratios in the control group and in the ATB group before or after treatment. Furthermore, according to multifactor analysis, the independent factors influencing the CD4/CD8 ratio overlapped with the risk factors for CD4 (Table 3). However, in patients with drug-resistant TB, as the number of CD8 T-cells decreased further, the corresponding increase in the CD4/CD8 ratio was statistically significant, suggesting that the observed depletion of CD8 T-cells may be related to the development of drug resistance. It is speculated that in addition to the drug resistance mechanism of Mtb itself, this phenomenon is mainly related to an insufficient number of CD8 T-cells, which leads to weakened secretion of granzyme and perforin, decreased cytolytic activity and the inability to clear intracellular pathogens [24]. Further study is needed to determine which occurs first, the decline in T-lymphocyte counts or the decline in activity after Mtb infection.
The results of this study demonstrated that lower CD4 and CD8 T-cell counts were associated with greater extent of lesions in the patients, suggesting that immune function declines with increasing severity of the disease. This finding differs from that of an earlier report [25], possibly due to differences in sample sizes, geographical environments, and ethnic backgrounds studied. No significant changes in CD4/CD8 ratios were detected, and in peripheral blood, CD4 and CD8 are more sensitive markers than the CD4/CD8 ratio. Changes in CD4 and CD8 T-cells were not associated with any concurrent extrapulmonary TB, and the influences of the changes in previously treated cases and in new cases were marginal. In the in-depth analysis, we found that the incidence of severe lesions was higher in previously treated cases (χ2 = 13.392, P < 0.0001; Supplementary Table 4). Thus, it is clear that CD4 and CD8 T-cell counts have certain value for predicting the severity of TB lesions in EDCT. However, severe lesions are also associated with a greater likelihood of treatment failure.
T-cell proliferation and gamma interferon synthesis decrease with age [26]. These changes are detrimental to the body's defenses and decrease the protective response against TB. Patients age 61 and over accounted for 36.73% (101/275) of the patients in the present study. The univariate analysis found that age was significantly negatively correlated with CD4 and CD8 T-cell counts, while the multivariate analysis showed that age was the main factor that negatively influenced CD8 T-cell count. The above findings indicate that a decrease in CD8 cytotoxic T-cell-mediated protective immunity against Mtb might cause an increase in the incidence of TB and reactivation of previous infection. The findings are consistent with the results of Chen et al. [27], who showed that CD8 T-cell depletion may result in Mtb reinfection.
Accumulating evidence suggests that platelets are the key effector cells in host regulation of inflammatory responses and that they contribute to the initiation and transmission of local and systemic inflammatory processes [28]. Platelets stimulate T-lymphocyte adhesion by secreting the key chemical inducer RANTES (regulated via activation, normal T-cell expressed and secreted, also known as CCL5), thereby regulating T-lymphocyte function [29]. The present study found that changes in the number of platelets were positively correlated with CD4 and CD8 T-cell counts. We were unable to retrieve any clinical data related to this finding from previous studies on ATB.
BMI, hemoglobin, PA, and albumin are indicators that reflect the nutritional status of patients. The negative effects of malnutrition on cellular immunity have been firmly established [30]. The underlying mechanisms may involve impaired antigen-presenting cell function [31] and inhibition of glucose metabolism-dependent T-cell activation [32, 33], both of which reduce the production of protective cytokines [34]. In tuberculosis patients, anemia is related to the overexpression of pro-inflammatory factors that inhibit erythropoietin and to changes in iron metabolism [35]. Due to the correlations of BMI and hemoglobin with CD4, BMI may be a potential indicator reflecting CD4 counts under limited resources [36,37,38]. These quantitative indicators demonstrate a positive correlation between malnutrition and immune status in TB, and it is possible that malnutrition increases the risk of immune impairment more robustly than does diabetes. Among the malnutrition indicators studied, the PA level was the most important. These findings suggest that the use of existing antituberculosis drugs and improving the nutritional status of the general population may be key TB control strategies.
The alpha-1 globulins mainly consist of alpha-1 antitrypsin (AAT), alpha-1-acid glycoprotein (AGP), alpha-1 lipoprotein, and alpha-fetoprotein. AAT and AGP are acute-phase response proteins [39, 40]. In ATB, alpha-1 globulin performs the functions of both AGP and AAT. In a study conducted by Zhang et al. the most significant differential protein peak in serum was identified as AGP [41], and the most important cell sources included alveolar macrophages and type II alveolar cells [42]. In the present study, the alpha-1 globulin level exceeded the upper limit of normal (4.36 g/L) in 58.26% (141/242) of the study sample, consistent with the results of recent studies [43, 44]. The multivariate analysis showed that alpha-1 globulin level was negatively correlated with quantitative changes in CD4 T-cell count. In contrast, the erythrocyte sedimentation rate, which is also a marker of the inflammatory response, was eliminated from the analysis due to its nonsignificant effect. Moreover, the alpha-1 globulin level was more important than both BMI and platelet count. These clinical data suggest that alpha-1 globulin can inhibit cell-mediated immune responses (Fig. 3d) and promote Mtb growth and disease progression [42]. Thus, the alpha-1 globulin level deserves further investigation as a potential marker.
Our research demonstrates that Mtb load is negatively correlated with the number of CD4 cells, and after treatment, T lymphocytes may increase, possibly because the expression of the Mtb immunodominant antigen depends on the level of virulence protein secreted by Mtb entering the cytoplasm [45]. These results are consistent with those of previous studies showing that high antigen levels consistently lead to T-cell failure [18, 46]. However, we found that among patients with high Mtb load (Mtb load ≥ 3 +), 18.82% (16/85) of patients still had CD4 ≥ 404 cells/μL, and 56.47% (48/85) of patients had CD8 ≥ 220 cells/μL. These patients did not show decreases in the number of lymphocytes, which may be related to the upregulation of the expression of T-cell inhibitory receptors (such as programmed death-1, T-cell immunoglobulin and mucin domain-containing protein-3, and lymphocyte-activated gene-3). These T-cell inhibitory receptors not only negatively regulate the proliferation of reactive cells but also affect their function and cause immune paralysis [47].
In patients with a history of previous treatment, the T-lymphocyte count was even lower. Although the statistical results were marginal, the duration of symptoms before admission was negatively correlated with the CD4 count, suggesting that the continued presence of bacterial antigens leads to lymphocyte failure. This finding is consistent with the importance of the timing of the immune response in human viral infectious diseases [48].
The main component of white blood cells (WBC) is neutrophils. Neutrophils can carry antigens from peripheral sites into lymph nodes and bone marrow and promote the generation of Th1, Th17, and CD8 memory responses [49,50,51]. The release of neutrophil extracellular traps (NETs) can reduce the activation threshold of T-cells [52] and promote the activation of naïve antigen-specific CD4 T-cells [53]. In addition, the production of granulocyte–macrophage colony-stimulating factors by T-cells during Mtb infection can enhance the host’s resistance to Mtb [54]. This study found a positive correlation of WBC with CD4 but no statistically significant correlation with CD8, suggesting that WBC is one of the activation factors affecting CD4 cells.
The main proteins in the beta-1 globulin fraction are transferrin, hemopexin, beta-lipoprotein, and complement C4. Transferrin and hemopexin are involved in the transport and storage of iron, and they often decline together with albumin during acute-phase reactions and malnutrition. Previously, Minchella et al. [55] found that low transferrin levels are a risk factor for the development of active tuberculosis in people with latent tuberculosis infection. In this study, all β1 globulin proteins had various effects on T lymphocytes, suggesting that β1 globulin may promote tuberculosis activities by acting on the body’s immune function via the following routes: transferrin and hemopexin may decrease, thereby reducing transferrin-Fe3+ entering the bone marrow; during Mtb infection, pathogens may obtain iron from host iron carrier proteins via the synthesis of high-affinity iron chelators and expression of the glyceraldehyde-3-phosphate dehydrogenase receptor on the cell surface [56]; the patient’s iron deficiency leads to inhibition of lymphocyte DNA synthesis, abnormal proliferation, and differentiation of T lymphocytes, and a reduced antibacterial effect of macrophages and granulocytes [57, 58]. β-Lipoprotein can decrease with the loss of plasma protein, and its final change depends on compensatory synthesis by hepatocytes, which is unpredictable. Complement C4 increases in inflammation and infection, and the increased complement C4 in this study may offset and mask the correlation between transferrin and T-lymphocyte count. The relationship between lymphocytes and transferrin warrants further study. Regulating the imbalance of iron in the body and blocking the ion supply of Mtb may be a potential strategy to rescue drug-resistant tuberculosis and improve the effectiveness of existing antituberculosis drugs in the future [59].
The above factors of Mtb load, duration of symptoms, WBC, hemoglobin, and beta-1 globulin showed statistical significance in the single-factor analysis but were not statistically significant in multifactor analysis, potentially due to their limited contributions or confounding factors.
The present study has several limitations. First, because it was an observational study, Berkson’s rate bias could not be excluded. For example, patients with mild conditions who were not hospitalized and patients who did not seek treatment were not included, resulting in a low number of cases with an image grading of “Minimal/Mild”. Second, for several variables, more than 10% of the data were missing. However, the missing data were less likely to affect the conclusions of our study due to the potential existence of nondifferential misclassification. Third, the patients included in our analysis were all confirmed ATB patients. Therefore, the results might not be suitable for clinical diagnosis or applicable to suspected TB cases. However, in ATB patients who are HIV-negative, we defined the specific level of the positive correlation between CD4 and CD8 T-cells and for the first time provided a report on the negative association between CD4 and CD8 T-cell counts and EDCT results.
Conclusion
In summary, examining T-lymphocyte subsets in ATB patients, especially CD4 and CD8 T-cells, is of great value in evaluating the immune function of the patients and assessing their disease status. The changes in the prevalence of the two subtypes of T-cells were negatively correlated with EDCT results, and the observed increase in the CD4/CD8 ratio suggests that other possible signs of drug resistance should be examined. For patients with hypoproteinemia or high alpha-1 globulin levels or patients of advanced age, immunological interventions in addition to anti-TB therapy can be beneficial and may help promote the development of host-directed therapies. However, these hypotheses require confirmation through in-depth, high-quality, randomized controlled trial studies.
References
Yao S, Huang D, Chen CY, Halliday L, Wang RC, Chen ZW. CD4+ T cells contain early extrapulmonary tuberculosis (TB) dissemination and rapid TB progression and sustain multieffector functions of CD8+ T and CD3– lymphocytes: mechanisms of CD4+ T cell immunity. J Immunol. 2014;192:2120–32.
Khan N, Vidyarthi A, Amir M, Mushtaq K, Agrewala JN. T-cell exhaustion in tuberculosis: pitfalls and prospects. Crit Rev Microbiol. 2017;43:133–41.
Sharma B, Upadhyay R, Dua B, Khan NA, Katoch VM, Bajaj B, et al. Mycobacterium tuberculosis secretory proteins downregulate T cell activation by interfering with proximal and downstream T cell signalling events. BMC Immunol. 2015;16:67.
Sande OJ, Karim AF, Li Q, Ding X, Harding CV, Rojas RE, et al. Mannose-capped lipoarabinomannan from Mycobacterium tuberculosis induces CD4+ T cell anergy via GRAIL. J Immunol. 2016;196:691–702.
Lin PL, Rutledge T, Green AM, Bigbee M, Fuhrman C, Klein E, et al. CD4 T cell depletion exacerbates acute Mycobacterium tuberculosis while reactivation of latent infection is dependent on severity of tissue depletion in cynomolgus macaques. AIDS Res Hum Retroviruses. 2012;28:1693–702.
Rozot V, Vigano S, Mazza-Stalder J, Idrizi E, Day CL, Perreau M, et al. Mycobacterium tuberculosis-specific CD8+ T cells are functionally and phenotypically different between latent infection and active disease. Eur J Immunol. 2013;43:1568–77.
Rozot V, Patrizia A, Vigano S, Mazza-Stalder J, Idrizi E, Day CL, et al. Combined use of Mycobacterium tuberculosis-specific CD4 and CD8 T-cell responses is a powerful diagnostic tool of active tuberculosis. Clin Infect Dis. 2015;60:432–7.
Athman JJ, Sande OJ, Groft SG, Reba SM, Nagy N, Wearsch PA, et al. Mycobacterium tuberculosis membrane vesicles inhibit T cell activation. J Immunol. 2017;198:2028–37.
Jiang H, Gong H, Zhang Q, Gu J, Liang L, Zhang J. Decreased expression of perforin in CD8+ T lymphocytes in patients with Mycobacterium tuberculosis infection and its potential value as a marker for efficacy of treatment. J Thorac Dis. 2017;9:1353–60.
Kumar Nathella P, Babu S. Influence of diabetes mellitus on immunity to human tuberculosis. Immunology. 2017;152:13–24.
Kumar NP, Moideen K, George PJ, Dolla C, Kumaran P, Babu S. Impaired cytokine but enhanced cytotoxic marker expression in Mycobacterium tuberculosis-induced CD8+ T cells in individuals with type 2 diabetes and latent Mycobacterium tuberculosis infection. J Infect Dis. 2015;213:866–70.
DiNardo AR, Mace EM, Lesteberg K, Cirillo JD, Mandalakas AM, Graviss EA, et al. Schistosome soluble egg antigen decreases Mycobacterium tuberculosis-specific CD4+ T-cell effector function with concomitant arrest of macrophage phago-lysosome maturation. J Infect Dis. 2016;214:479–88.
Kugler DG, Flomerfelt FA, Costa DL, Laky K, Kamenyeva O, Mittelstadt PR, et al. Systemic toxoplasma infection triggers a long-term defect in the generation and function of naive T lymphocytes. J Exp Med. 2016;213:3041–56.
Wang J, Dai Y, Liu J, Yin Y, Pei H. MTB-specific lymphocyte responses are impaired in tuberculosis patients with pulmonary cavities. Eur J Med Res. 2017;22:4.
Kaplan G, Post FA, Moreira AL, Wainwright H, Kreiswirth BN, Tanverdi M, et al. Mycobacterium tuberculosis growth at the cavity surface: a microenvironment with failed immunity. Infect Immun. 2003;71:7099–108.
Gao WW, Wang Y, Zhang XR, Yin CY, Hu CM, Tian M, et al. Levels of 1,25(OH)2D3 for patients with pulmonary tuberculosis and correlations of 1,25(OH)2D3 with the clinical features of TB. J Thorac Dis. 2014;6:760–4.
Zeng J, Wu G, Yang W, Gu X, Liang W, Yao Y, et al. A serum vitamin D level %3c25nmol/l pose high tuberculosis risk: a meta-analysis. PLoS ONE. 2015;10:e0126014.
Day CL, Abrahams DA, Lerumo L, Janse van Rensburg E, Stone L, O'rie T, et al. Functional capacity of Mycobacterium tuberculosis-specific T cell responses in humans is associated with mycobacterial load. J Immunol. 2011;187:2222–32.
National Health Commission of the People’s Republic of China. Diagnostic criteria and principles of management of infectious pulmonary tuberculosis (GB15987-1995). https://www.nhc.gov.cn/wjw/s9491/201212/34117.shtml (1995). Accessed 8 Jan 2019.
American Diabetes Association. Standards of medical care in diabetes-2013. Diabetes Care. 2013;36:S11–S66.
Seaton A, Seaton D, Leitch AG. Crofton and Douglas’s respiratory diseases. In: Leitch AG, editor. Pulmonary tuberculosis: clinical features. 4th ed. Oxford: Blackwell Press; 1989. p. 409–410.
Hazra A, Gogtay N. Biostatistics series module 6: correlation and linear regression. Indian J Dermatol. 2016;61:593–601.
Jasenosky LD, Scriba TJ, Hanekom WA, Goldfeld AE. T cells and adaptive immunity to Mycobacterium tuberculosis in humans. Immunol Rev. 2015;264:74–877.
Kamath A, Woodworth JS, Behar SM. Antigen-specific CD8+ T cells and the development of central memory during Mycobacterium tuberculosis infection. J Immunol. 2006;177:6361–9.
Uppal SS, Tewari SC, Verma S, Dhot PS. Comparison of CD4 and CD8 lymphocyte counts in HIV-negative pulmonary TB patients with those in normal blood donors and the effect of antitubercular treatment: hospital-based flow cytometric study. Cytometry B Clin Cytom. 2004;61:20–6.
Menon S, Rossi R, Nshimyumukiza L, Wusiman A, Zdraveska N, Eldin MS. Convergence of a diabetes mellitus, protein energy malnutrition, and TB epidemic: the neglected elderly population. BMC Infect Dis. 2016;16:361.
Chen CY, Huang D, Wang RC, Shen L, Zeng G, Yao S, et al. A critical role for CD8 T cells in a nonhuman primate model of tuberculosis. PLoS Pathog. 2009;5:e1000392.
Fox KA, Kirwan DE, Whittington AM, Krishnan N, Robertson BD, Gilman RH, et al. Platelets regulate pulmonary inflammation and tissue destruction in tuberculosis. Am J Respir Crit Care Med. 2018;198:245–55.
Nouailles G, Dorhoi A, Koch M, Zerrahn J, Weiner J 3rd, Faé KC, et al. CXCL5-secreting pulmonary epithelial cells drive destructive neutrophilic inflammation in tuberculosis. J Clin Invest. 2014;124:1268–82.
McMurray DN, Cegielski JP. The influence of nutrition on the risk and outcomes of tuberculosis. In: of South Africa Consensus Panel on Nutrition, HIV/AIDS, and TB, editor. HIV/AIDS, TB, and Nutrition: Scientific inquiry into the nutritional influences on human immunity with special reference to HIV infection and active TB in South Africa. Pretoria: Academy of Science of South Africa. 2007. pp. 153–69.
Ibrahim MK, Zambruni M, Melby CL, Melby PC. Impact of childhood malnutrition on host defense and infection. Clin Microbiol Rev. 2017;30:919–71.
Saucillo DC, Gerriets VA, Sheng J, Rathmell JC, Maciver NJ. Leptin metabolically licenses T cells for activation to link nutrition and immunity. J Immunol. 2013;192:136–44.
Jacobs SR, Herman CE, Maciver NJ, Wofford JA, Wieman HL, Hammen JJ, et al. Glucose uptake is limiting in T cell activation and requires CD28-mediated Akt-dependent and independent pathways. J Immunol. 2008;180:4476–86.
Anuradha R, Munisankar S, Bhootra Y, Kumar NP, Dolla C, Kumaran P, et al. coexistent malnutrition is associated with perturbations in systemic and antigen-specific cytokine responses in latent tuberculosis infection. Clin Vaccine Immunol. 2016;23:339–45.
Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352:1011–23.
Koethe JR, Jenkins CA, Shepherd BE, Stinnette SE, Sterling TR. An optimal body mass index range associated with improved immune reconstitution among HIV-infected adults initiating antiretroviral therapy. Clin Infect Dis. 2011;53:952–60.
Kwantwi LB, Tunu BK, Boateng D, Quansah DY. Body mass index, haemoglobin, and total lymphocyte count as a surrogate for CD4 count in resource limited settings. J Biomark. 2017;2017:7907352.
Abuaf N, Leynadier F, Luce H, Dry J. Haemoglobin content of multilayer E rosettes is a parameter of activated human T lymphocytes. Clin Exp Immunol. 1981;44:145–51.
Almeida ML, Barbieri MA, Gurgel RQ, Abdurrahman ST, Baba UA, Hart CA, et al. Alpha1-acid glycoprotein and alpha1-antitrypsin as early markers of treatment response in patients receiving the intensive phase of tuberculosis therapy. Trans R Soc Trop Med Hyg. 2009;103:575–80.
Song SH, Han M, Choi YS, Dan KS, Yang MG, Song J, et al. Proteomic profiling of serum from patients with tuberculosis. Ann Lab Med. 2014;34:345–53.
Zhang J, Wu X, Shi L, Liang Y, Xie Z, Yang Y, et al. Diagnostic serum proteomic analysis in patients with active tuberculosis. Clin Chim Acta. 2012;413:883–7.
Martìnez Cordero E, Gonzàlez MM, Aguilar LD, Orozco EH, Hernàndez Pando R. Alpha-1-acid glycoprotein, its local production and immunopathological participation in experimental pulmonary tuberculosis. Tuberculosis (Edinb). 2008;88:203–11.
Santos VS, Goletti D, Kontogianni K, Adams ER, Molina-Moya B, Dominguez J, et al. Acute phase proteins and IP-10 as triage tests for the diagnosis of tuberculosis: systematic review and meta-analysis. Clin Microbiol Infect. 2019;25:169–77.
Sun H, Pan L, Jia H, Zhang Z, Gao M, Huang M, et al. Label-free quantitative proteomics identifies novel plasma biomarkers for distinguishing pulmonary tuberculosis and latent infection. Front Microbiol. 2018;9:1267.
van der Wel N, Hava D, Houben D, Fluitsma D, van Zon M, Pierson J, et al. M. tuberculosis and M. leprae translocate from the phagolysosome to the cytosol in myeloid cells. Cell. 2007;129:1287–98.
Mueller SN, Ahmed R. High antigen levels are the cause of T cell exhaustion during chronic viral infection. Proc Natl Acad Sci USA. 2009;106:8623–8.
Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29–37.
Li Q, Skinner PJ, Ha SJ, Duan L, Mattila TL, Hage A, et al. Visualizing antigen-specific and infected cells in situ predicts outcomes in early viral infection. Science. 2009;323:1726–9.
Abi Abdallah DS, Egan CE, Butcher BA, Denkers EY. Mouse neutrophils are professional antigen-presenting cells programmed to instruct Th1 and Th17 T-cell differentiation. Int Immunol. 2011;23:317–26.
Duffy D, Perrin H, Abadie V, Benhabiles N, Boissonnas A, Liard C, et al. Neutrophils transport antigen from the dermis to the bone marrow, initiating a source of memory CD8+ T cells. Immunity. 2012;37:917–29.
Kalyan S, Kabelitz D. When neutrophils meet T cells: beginnings of a tumultuous relationship with underappreciated potential. Eur J Immunol. 2014;44:627–33.
Tillack K, Breiden P, Martin R, Sospedra M. T lymphocyte priming by neutrophil extracellular traps links innate and adaptive immune responses. J Immunol. 2012;188:3150–9.
Blomgran R, Ernst JD. Lung neutrophils facilitate activation of naive antigen-specific CD4+ T cells during Mycobacterium tuberculosis infection. J Immunol. 2011;186:7110–9.
Rothchild AC, Stowell B, Goyal G, Nunes-Alves C, Yang Q, Papavinasasundaram K, et al. Role of granulocyte-macrophage colony-stimulating factor production by T cells during Mycobacterium tuberculosis infection. mBio. 2017;8:e01514–e1517.
Minchella PA, Donkor S, McDermid JM, Sutherland JS. Iron homeostasis and progression to pulmonary tuberculosis disease among household contacts. Tuberculosis (Edinb). 2015;95:288–93.
Malhotra H, Patidar A, Boradia VM, Kumar R, Nimbalkar RD, Kumar A, et al. Mycobacterium tuberculosis Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH) functions as a receptor for human lactoferrin. Front Cell Infect Microbiol. 2017;7:245.
Hassan TH, Badr MA, Karam NA, Zkaria M, El Saadany HF, Abdel Rahman DM, et al. Impact of iron deficiency anemia on the function of the immune system in children. Medicine (Baltimore). 2016;95:e5395.
de Pontual L. Iron and susceptibility to infections. Arch Pediatr. 2017;24:5S14–17.
Pal R, Hameed S, Kumar P, Singh S, Fatima Z. Understanding lipidomic basis of iron limitation induced chemosensitization of drug-resistant Mycobacterium tuberculosis. 3 Biotech. 2019;9:122.
Acknowledgements
The authors would like to thank their colleagues in the hematology laboratory for providing detection results for T-lymphocyte subsets. American Journal Experts (Durham, North Carolina) provided assistance in translating the manuscript.
Funding
This study was supported by the Key Research and Development Program of Shaanxi (Program No. 2020SF-113).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical approval
The present study was approved by the Ethics Committee of Ankang Central Hospital (ECACH-2016012). Because the study was a case-based retrospective study that did not put patients at risk, the requirement for informed consent was waived.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, K., Ran, R., Jiang, Z. et al. Changes in T-lymphocyte subsets and risk factors in human immunodeficiency virus-negative patients with active tuberculosis. Infection 48, 585–595 (2020). https://doi.org/10.1007/s15010-020-01451-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-020-01451-2