Abstract
Background
To establish the prognostic and discriminative value of the pro-atrial natriuretic peptide (pro-ANP) level in patients with severe sepsis or septic shock.
Patients and methods
An observational and prospective study was conducted on 50 critically ill patients with severe sepsis or septic shock. Measurements of the level of procalcitonin (PCT) and mid-regional pro-ANP were determined in the serum of patients with commercially available immunoluminometric tests.
Results
The median pro-ANP level was significantly higher in non-survivors than in survivors (P < 0.05) on all consecutive days. No significant differences in the pro-ANP levels were observed in patients with severe sepsis and septic shock. There was a strong correlation between the PCT and pro-ANP levels on admission in non-survivors and in septic shock patients (r = 0.56, P = 0.007 and r = 0.43, P = 0.02, respectively).
Conclusions
pro-ANP evaluated in severe sepsis and septic shock patients is a valuable prognostic biomarker, but, in contrast to PCT, which is routinely used as a diagnostic marker of severe sepsis and septic shock, it does not possess diagnostic and discriminative value.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Immune inflammatory reactions, which are responses to infection, are essential to the body’s protective mechanisms. The often excessive and uncontrolled course of such reactions is one of the crucial factors influencing multiple organ dysfunction syndrome (MODS), which impacts intensive care unit (ICU) patient mortality. According to the definition from 1992 [1], there are three clinical forms of response to infection: sepsis, severe sepsis, and septic shock. They are differentiated by the presence and degree of the intensity of cardiovascular dysfunction resulting from the extensity of the inflammatory response [2]. Severe sepsis is defined as sepsis with one or more organ dysfunctions or tissue hypoperfusion [3]. Septic shock is characterized by the most advanced form of circulatory system failure, and is defined as severe sepsis with persisting hypotension, despite adequate fluid resuscitation or blood lactate concentration ≥4 mmol/L [3]. The pathophysiology of severe sepsis and septic shock at the cellular level is associated with an advanced form of microcirculatory and mitochondrial distress syndrome (MMDS). It involves endothelium dysfunction with apoptosis and coagulation disturbances, resulting in global tissue hypoxia [4, 5]. Atrial natriuretic peptide (ANP), consisting of 28 amino acids, is a peptide hormone and a member of the natriuretic peptide family. Its conservative part is composed of 17 amino acids that form a ring structure with disulfide bonds and determine the biological features of ANP. ANP with a half-life of 3–4 min is cleared out rapidly from the circulation. pro-ANP, composed of 126 amino acids, is the precursor of ANP and is the primary form of storing ANP in atrial cardiomyocytes. The N-terminal part of pro-ANP (1–98) has a longer half-life (60–120 min) than ANP. ANP possesses diuretic and natriuretic properties, resulting from the direct inhibition of sodium absorption in the renal collecting duct. ANP decreases blood pressure, modulates endothelium permeability, and has an effect on the volume and pressure homeostasis in the circulatory system [6]. There are two transmembrane receptors, NPR-A and NPR-C (a clearance receptor), which regulate ANP activity [7, 8]. Natriuretic peptides participate in the innate and acquired immunological response [9]. ANP has been shown to have a modulating effect on macrophage function and the priming of polymorphonuclear neutrophils [10].
ANP interferes with both the mitogen-activated protein kinase (MAPK) network and transcription factors, mainly, NF-κB [11]. Procalcitonin is a 13-kDa prohormone of calcitonin. Carboxyl-terminal (CT) peptides (procalcitonin [PCT] included) have a common origin on the CALC-1 gene on chromosome 11 [12]. It has been suggested that PCT is a prototype of hormokine mediators. The definition of hormokine includes the cytokine-like activity of hormones during inflammation and infection [13]. During the response to infectious stimuli, there is only a limited and transient release of PCT from macrophages and monocytes. LPS, IL-1β, and TNF-α promote the tissue-wide induction of CT mRNA, with the result that PCT is secreted into the circulation.
The half-life of PCT is about 22–35 h [14]. Under physiological conditions, the level of PCT is lower than <0.5 ng/ml. It has been stated that PCT should be used as a diagnostic marker to monitor the course of severe sepsis and septic shock and antimicrobial therapy [15, 16].
Patients and methods
Patients
Fifty patients with severe sepsis or septic shock admitted to the Department of Anesthesiology and Intensive Therapy of Wroclaw Medical University, Poland, were enrolled in the study. Patients with cardiogenic shock and other acute circulatory failure not induced by infection were excluded from the study. Critically ill patients were divided into the following subgroups: survivors (n = 26) and non-survivors (n = 24), and severe sepsis (n = 19) and septic shock (n = 31). The mean age and range (in years) of survivors and non-survivors was 51 (18–88) and 60.6 (19–91) (P = 0.057), respectively, and in the severe sepsis and septic shock groups, they were 47.8 (18–80) and 60.7 (21–91) (P = 0.02), respectively.
The diagnosis of severe sepsis or septic shock was performed according to the 2001 Consensus Conference Criteria [3].
Infection was confirmed with microbiological tests, radiological analysis, and surgical procedures. In questionable cases, the PCT level was used as confirmation of the presence of infection.
This study was observational and prospective. Table 1 shows the detailed microbiological results and information about the sources of infection. The status of clinical patients was assessed with the Acute Physiology and Chronic Health Evaluation II (APACHE II) score [17] on admission to the ICU, and the extent of multiple organ failure was evaluated using the Sequential Organ Failure Assessment (SOFA) score [18] on admission, and on the 2nd, 3rd, and 5th days. All patients were treated according to accepted standards for severe sepsis and septic shock (antimicrobial therapy, mechanical ventilation, fluid resuscitation, vasopressor therapy). All patients had been receiving empirical antibiotic therapy on admission, which was modified according to ongoing microbiological and laboratory results. The ICU mortality was 48%.
The prognostic and discriminative value of pro-ANP levels was evaluated in patients diagnosed with severe sepsis and septic shock.
The control group included 20 healthy volunteers, comprising 15 females and 5 males (mean age 36.4 years, range 22–56 years), who were recruited from the ICU staff.
Ethical considerations
The study was approved by the Medical Ethics Committee of Wroclaw Medical University and informed consent was obtained from the patients or their legal representatives.
Laboratory measurements
Blood was drawn on admission, and on the 2nd, 3rd, and 5th days. In nine cases, samples from the 5th day were not available (the patients had died or had been discharged earlier). Serum was obtained after 15 min of clotting at room temperature and the blood was centrifuged (10 min, 720g). Serum samples were aliquoted and stored at −80°C to analyze the pro-ANP and PCT concentrations. All measurements were performed with commercially available immunoluminometric tests from BRAHMS Diagnostica GmbH (Hennigsdorf, Germany), according to the manufacturer’s instructions.
Statistical methods
The normality of the distribution was estimated by the Kolmogorov–Smirnov test. The data were analyzed with a non-parametric test (Mann–Whitney U-test) to compare the two groups. The APACHE II and SOFA score values are presented as the mean ± standard deviation (SD). A P value ≤0.05 was considered to be statistically significant. Correlation analysis with continuous data was conducted using Pearson’s correlation test (r).
All analyses were performed using the STATISTICA data analysis software system, version 9.1 (StatSoft Inc., 2010, http://www.statsoft.com).
Results
Survivors and non-survivors
As an estimation of the patients’ clinical status, the mean value of the APACHE II score on admission in survivors and non-survivors was 18.3 and 27, respectively (Table 2). The value of the SOFA score was significantly lower in survivors than in non-survivors on all the analyzed days (Table 2).
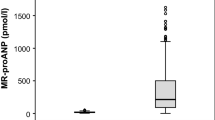
The pro-ANP level was significantly lower in survivors than non-survivors on all the analyzed days (Table 3).
In the receiver operating characteristic (ROC) curve analysis of survival, on the day of admission, the cut-off value was 220.58 pmol/L and area under the curve (AUC) = 0.72, the sensitivity was 0.917, and the specificity was 0.538 (Fig. 1a). On the 2nd therapy day, the cut-off value was 262.5 pmol/L and AUC = 0.699, the sensitivity was 0.75, and the specificity was 0.654 (Fig. 1b). On the 3rd therapy day, the cut-off value was 409.94 pmol/L and AUC = 0.673, the sensitivity was 0.591, and the specificity was 0.84 (Fig. 1c). On the 5th therapy day, the cut-off value was 270.34 pmol/L and AUC = 0.789, the sensitivity was 0.789, and the specificity was 0.76 (Fig. 1d).
The receiver operating characteristic (ROC) curve of the pro-atrial natriuretic peptide (pro-ANP) level of survival analysis on the 1st day with a cut-off value of 220.58 pmol/L (area under the curve [AUC] = 0.72, sensitivity 0.917, and specificity 0.538) (a); 2nd day with a cut-off value of 262.5 pmol/L (AUC = 0.699, sensitivity 0.75, and specificity 0.654) (b); 3rd day with a cut-off value of 409.94 pmol/L (AUC = 0.673, sensitivity 0.591, and specificity 0.84) (c); 5th day with a cut-off value of 270.34 pmol/L (AUC = 0.789, sensitivity 0.789, and specificity 0.76) (d)
Severe sepsis and septic shock
On admission, there was a statistically significant difference in the APACHE II score value of both groups (P = 0.05). The differences in the SOFA score value were statistically significant in the course of the study, except for the 5th day (Table 4).
There was no statistically significant difference in the pro-ANP level between the groups (Table 5).
In the ROC curve analysis of the diagnosis, on the day of admission, the cut-off value was 215 pmol/L and AUC = 0.59, the sensitivity was 0.81, and the specificity was 0.47 (Fig. 2a). On the 2nd therapy day, the cut-off value was 258 pmol/L and AUC = 0.548, the sensitivity was 0.61, and the specificity was 0.58 (Fig. 2b). On the 3rd therapy day, the cut-off value was 162 pmol/L and AUC = 0.594, the sensitivity was 0.80, and the specificity was 0.47 (Fig. 2c). On the 5th therapy day, the cut-off value was 143 pmol/L and AUC = 0.632, the sensitivity was 0.81, and the specificity was 0.53 (Fig. 2d).
The ROC curve of the pro-ANP level of the diagnosis on the 1st day with a cut-off value of 215 pmol/L (AUC = 0.59, sensitivity 0.81, and specificity 0.47) (a); 2nd day with a cut-off value of 258 pmol/L (AUC = 0.548, sensitivity 0.61, and specificity 0.58) (b); 3rd day with a cut-off value of 162 pmol/L (AUC = 0.594, sensitivity 0.80, and specificity 0.47) (c); 5th day with a cut-off value of 143 pmol/L (AUC = 0.632, sensitivity 0.81, and specificity 0.53) (d)
There was a strong correlation between the PCT and pro-ANP levels on admission in non-survivors and in septic shock patients (r = 0.56, P = 0.007 (Fig. 3a) and r = 0.43, P = 0.02 (Fig. 3b), respectively).
Discussion
This study was performed in order to determine the prognostic and discriminative properties of pro-ANP in critically ill patients. In the study, the level of pro-ANP was lower in survivors than non-survivors, with statistical significance reached on all the study days; this was similar to the results obtained by Vazquez et al. [19]. A decline in the pro-ANP level was observed starting from the 2nd day in survivors. Similar results were presented by Morgenthaler et al. [20] and Boeck et al. [21], but were in contrary to those of Prucha et al. [22]. In Prucha et al.’s study, the pro-ANP level did not show any tendencies to increase or decrease. There was a correlation between the levels of PCT and pro-ANP on the day of admission in the non-survivors and septic shock groups. In Morgenthaler et al. [20] study, the circulating pro-ANP levels showed a similar increase when categorized on the increasing PCT in all the study groups.
The highest values of pro-ANP were observed in non-survivors and was directly associated with the highest APACHE II and SOFA score results, reflecting the clinical status and progress of multiple organ failure in this group. There was a correlation between the SOFA value and pro-ANP level in the non-survivors and septic shock group on the 1st day. APACHE II and SOFA score values were lower in the survivors group on admission.
The pro-ANP level, which was not significant for severe sepsis and septic shock subgroups, did not reflect statistical significance in the APACHE II and SOFA score results. These results were similar to Morgenthaler et al. study [20], where there were no statistically significant differences in the pro-ANP levels between the severe sepsis and septic shock groups. All those results indicate that pro-ANP measured in severe sepsis or septic shock patients is a valuable prognostic biomarker without diagnostic and discriminative value, which is in contrast to PCT [23, 24]. Boeck et al. [21] found that patients with the highest pro-ANP quartile at ventilator-associated pneumonia (VAP) onset were at increased risk for death, and pro-ANP was identified as the best predictor of survival, followed by the SOFA and Simplified Acute Physiology Score (SAPS) II values.
Morgenthaler et al. [20] did not observe any significant differences in the pro-ANP levels between survivors and non-survivors with no infection. This suggests that pro-inflammatory factors like TNF-α and IL-6 have a greater influence on the release of pro-ANP than the hemodynamic changes linked to circulatory dysfunction that is essential for severe sepsis or septic shock [25].
The question as to why the level of pro-ANP is similar in such clinically differentiated conditions like severe sepsis and septic shock is still open for discussion and further study.
References
American College of Chest Physicians/Society of Critical Care Medicine Consensus. Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–74.
Hollenberg SM, Ahrens TS, Annane D, Astiz ME, Chalfin DB, Dasta JF, Heard SO, Martin C, Napolitano LM, Susla GM, Totaro R, Vincent JL, Zanotti-Cavazzoni S. Practice parameters for hemodynamic support of sepsis in adult patients: 2004 update. Crit Care Med. 2004;32:1928–48.
Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G; SCCM/ESICM/ACCP/ATS/SIS. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–6.
Vallet B. Bench-to-bedside review: endothelial cell dysfunction in severe sepsis: a role in organ dysfunction? Crit Care. 2003;7:130–8.
Ince C. Microcirculation in distress: a new resuscitation end point? Crit Care Med. 2004;32:1963–4.
Nishikimi T, Maeda N, Matsuoka H. The role of natriuretic peptides in cardioprotection. Cardiovasc Res. 2006;69:318–28.
Misono KS. Natriuretic peptide receptor: structure and signaling. Mol Cell Biochem. 2002;230:49–60.
Misono KS, Ogawa H, Qiu Y, Ogata CM. Structural studies of the natriuretic peptide receptor: a novel hormone-induced rotation mechanism for transmembrane signal transduction. Peptides. 2005;26:957–68.
Vollmar AM. The role of atrial natriuretic peptide in the immune system. Peptides. 2005;26:1086–94.
Wiedermann CJ, Niedermühlbichler M, Braunsteiner H. Priming of polymorphonuclear neutrophils by atrial natriuretic peptide in vitro. J Clin Invest. 1992;89:1580–6.
Ladetzki-Baehs K, Keller M, Kiemer AK, Koch E, Zahler S, Wendel A, Vollmar AM. Atrial natriuretic peptide, a regulator of nuclear factor-κB activation in vivo. Endocrinology. 2007;148:332–6.
Becker KL, Nylén ES, White JC, Müller B, Snider RH Jr. Clinical review 167: Procalcitonin and the calcitonin gene family of peptides in inflammation, infection, and sepsis: a journey from calcitonin back to its precursors. J Clin Endocrinol Metab. 2004;89:1512–25.
Müller B, Becker KL. Procalcitonin: how a hormone became a marker and mediator of sepsis. Swiss Med Wkly. 2001;131:595–602.
Reinhart K, Karzai W, Meisner M. Procalcitonin as a marker of the systemic inflammatory response to infection. Intensive Care Med. 2000;26:1193–200.
Uzzan B, Cohen R, Nicolas P, Cucherat M, Perret GY. Procalcitonin as a diagnostic test for sepsis in critically ill adults and after surgery or trauma: a systematic review and meta-analysis. Crit Care Med. 2006;34:1996–2003.
Hochreiter M, Köhler T, Schweiger AM, Keck FS, Bein B, von Spiegel T, Schroeder S. Procalcitonin to guide duration of antibiotic therapy in intensive care patients: a randomized prospective controlled trial. Crit Care. 2009;13:R83. doi:10.1186/cc7903.
Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–29.
Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, Reinhart CK, Suter PM, Thijs LG. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22:707–10.
Vazquez M, Jockers K, Christ-Crain M, Zimmerli W, Müller B, Schuetz P. MR-pro-atrial natriuretic peptide (MR-proANP) predicts short- and long-term outcomes in respiratory tract infections: a prospective validation study. Int J Cardiol. 2010. Nov 18. [Epub ahead of print]
Morgenthaler NG, Struck J, Christ-Crain M, Bergmann A, Müller B. Pro-atrial natriuretic peptide is a prognostic marker in sepsis, similar to the APACHE II score: an observational study. Crit Care. 2005;9:R37–45.
Boeck L, Eggimann P, Smyrnios N, Pargger H, Thakkar N, Siegemund M, Marsch S, Rakic J, Tamm M, Stolz D. Midregional pro-atrial natriuretic peptide and procalcitonin improve survival prediction in VAP. Eur Respir J. 2011;37:595–603. doi: 10.1183/09031936.00023810.
Průcha M, Zazula R, Dubská L, Sedláčková L, Kavka B. Pro-atriální natriuretický peptid u pacientů v sepsi, těžké sepsi a septickém šoku. Klin Biochem Metab. 2007;15:127–31.
Clec’h C, Ferriere F, Karoubi P, Fosse JP, Cupa M, Hoang P, Cohen Y. Diagnostic and prognostic value of procalcitonin in patients with septic shock. Crit Care Med. 2004;32:1166–9.
Schuetz P, Christ-Crain M, Thomann R, Falconnier C, Wolbers M, Widmer I, Neidert S, Fricker T, Blum C, Schild U, Regez K, Schoenenberger R, Henzen C, Bregenzer T, Hoess C, Krause M, Bucher HC, Zimmerli W, Mueller B; ProHOSP Study Group. Effect of procalcitonin-based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA. 2009;302:1059–66.
Witthaut R, Busch C, Fraunberger P, Walli A, Seidel D, Pilz G, Stuttmann R, Speichermann N, Verner L, Werdan K. Plasma atrial natriuretic peptide and brain natriuretic peptide are increased in septic shock: impact of interleukin-6 and sepsis-associated left ventricular dysfunction. Intensive Care Med. 2003;29:1696–702.
Acknowledgments
The present study was financially supported by Wroclaw Medical University grant number 1430.
Conflict of interest
None.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Lipinska-Gediga, M., Mierzchala, M. & Durek, G. Pro-atrial natriuretic peptide (pro-ANP) level in patients with severe sepsis and septic shock: prognostic and diagnostic significance. Infection 40, 303–309 (2012). https://doi.org/10.1007/s15010-011-0235-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-011-0235-0