Abstract
Background:
Hepatic fibrosis (HF) is a histopathological change in the process of long-term liver injury caused by cytokine secretion and internal environment disturbance, resulting in excessive liver repair and fiber scar. Nogo-B protein is widely distributed in peripheral tissues and organs and can regulate the migration of endothelial cells by activating TGF-β1 in vascular remodeling after injury. Nogo-B has been shown to promote organ fibrosis. This study was to determine the role of Nogo-B in HF.
Methods:
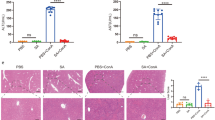
An HF model was built by intraperitoneal injections with 20% carbon tetrachloride. Localization of Nogo-B was detected by FISH. The interaction between Nogo-B and BACE1 was confirmed by Co-IP. Autophagy flux was analyzed using tandem mRFP-GFP-LC3 fluorescence microscopy, electron microscopy, and western blotting. Detection of serum AST and ALT and H&E staining were utilized to detect the degree of liver injury. The HF was evaluated by Masson trichromatic staining. RT-qPCR, western blotting, and immunofluorescence were employed to detect relevant indicators.
Results:
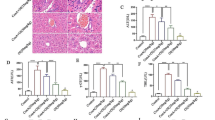
Reducing Nogo-B suppressed AST and ALT levels, the accumulation of collagen I and α-SMA, and expressions of pro-fibrotic genes in mouse liver. BACE1 was a potential downstream target of Nogo-B. Nogo-B was upregulated in TGF-β1-activated hepatic stellate cells (HSCs). Knocking down Nogo-B caused the downregulation of pro-fibrotic genes and inhibited viability of HSCs. Nogo-B knockdown prevented CCL4-induced fibrosis, accompanied by downregulation of extracellular matrix. Nogo-B inhibited HSC autophagy and increased lipid accumulation. BACE1 knockdown inhibited HSC autophagy and activation in LX-2 cells.
Conclusion:
Nogo-B knockdown prevents HF by directly inhibiting BACe1-mediated autophagy.








Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
References
Friedman SL, Pinzani M. Hepatic fibrosis 2022: unmet needs and a blueprint for the future. Hepatology. 2022;75:473–88.
Matsuda M, Seki E. Hepatic stellate cell-macrophage crosstalk in liver fibrosis and carcinogenesis. Semin Liver Dis. 2020;40:307–20.
Liu Yi, Que KT, Yang PF. TREM2 promotes liver fibrosis by enhancing hepatic stellate cell endocytosis and activation via phosphorylating ITGAV. J Biol Regul Homeost Agents. 2023;37:3693–704.
Khanam A, Saleeb PG, Kottilil S. Pathophysiology and treatment options for hepatic fibrosis: can it be completely cured? Cells. 2021;10:1097.
Parola M, Pinzani M. Liver fibrosis: pathophysiology, pathogenetic targets and clinical issues. Mol Aspects Med. 2019;65:37–55.
Rao J, Cheng F, Zhou H, Yang W, Qiu J, Yang C, et al. Nogo-B is a key mediator of hepatic ischemia and reperfusion injury. Redox Biol. 2020;37:101745.
Oertle T, Schwab ME. Nogo and its paRTNers. Trends Cell Biol. 2003;13:187–94.
Teng FY, Tang BL. Cell autonomous function of Nogo and reticulons: The emerging story at the endoplasmic reticulum. J Cell Physiol. 2008;216:303–8.
Zhang Y, Huo W, Wen Y, Li H. Silencing Nogo-B receptor inhibits penile corpus cavernosum vascular smooth muscle cell apoptosis of rats with diabetic erectile dysfunction by down-regulating ICAM-1. PLoS One. 2019;14:e0220715.
Dong C, Liu Y, Jiang K, Wang H, Qu W, Zhang C, et al. The Nogo-B receptor promotes human hepatocellular carcinoma cell growth via the Akt signal pathway. J Cell Biochem. 2018;119:7738–46.
Wen M, Men R, Yang Z, Dan X, Wu W, Liu X, et al. The value of circulating Nogo-B for evaluating hepatic functional reserve in patients with cirrhosis. Dis Markers. 2015;2015:419124.
Yang Q, Zhang C, Xie H, Tang L, Liu D, Qiu Q, et al. Silencing Nogo-B improves the integrity of blood-retinal barrier in diabetic retinopathy via regulating Src, PI3K/Akt and ERK pathways. Biochem Biophys Res Commun. 2021;581:96–102.
Tadokoro KS, Rana U, Jing X, Konduri GG, Miao QR, Teng RJ. Nogo-B receptor modulates pulmonary artery smooth muscle cell function in developing lungs. Am J Respir Cell Mol Biol. 2016;54:892–900.
Hernandez-Diaz I, Pan J, Ricciardi CA, Bai X, Ke J, White KE, et al. Overexpression of circulating soluble Nogo-B improves diabetic kidney disease by protecting the vasculature. Diabetes. 2019;68:1841–52.
Li YK, Xie YJ, Wu DC, Long SL, Tang S, Mo ZC. Nogo-B receptor in relevant carcinoma: Current achievements, challenges and aims (Review). Int J Oncol. 2018;53:1827–35.
Zhao D, Xue C, Yang Y, Li J, Wang X, Chen Y, et al. Lack of Nogo-B expression ameliorates PPARγ deficiency-aggravated liver fibrosis by regulating TLR4-NF-κB-TNF-α axis and macrophage polarization. Biomed Pharmacother. 2022;153:113444.
Mallat A, Lodder J, Teixeira-Clerc F, Moreau R, Codogno P, Lotersztajn S. Autophagy: a multifaceted partner in liver fibrosis. Biomed Res Int. 2014;2014:869390.
Cadwell K. Crosstalk between autophagy and inflammatory signalling pathways: balancing defence and homeostasis. Nat Rev Immunol. 2016;16:661–75.
Antonioli M, Di Rienzo M, Piacentini M, Fimia GM. Emerging mechanisms in initiating and terminating autophagy. Trends Biochem Sci. 2017;42:28–41.
Ba L, Gao J, Chen Y, Qi H, Dong C, Pan H, et al. Allicin attenuates pathological cardiac hypertrophy by inhibiting autophagy via activation of PI3K/Akt/mTOR and MAPK/ERK/mTOR signaling pathways. Phytomedicine. 2019;58:152765.
Varshney P, Saini N. PI3K/AKT/mTOR activation and autophagy inhibition plays a key role in increased cholesterol during IL-17A mediated inflammatory response in psoriasis. Biochim Biophys Acta Mol Basis Dis. 2018;1864:1795–803.
Filali-Mouncef Y, Hunter C, Roccio F, Zagkou S, Dupont N, Primard C, et al. The ménage à trois of autophagy, lipid droplets and liver disease. Autophagy. 2022;18:50–72.
Xu J, Wu ZS, Wang Z, Le J, Zheng T, Jia L. Autonomous assembly of ordered metastable DNA nanoarchitecture and in situ visualizing of intracellular microRNAs. Biomaterials. 2017;120:57–65.
Feng T, Tammineni P, Agrawal C, Jeong YY, Cai Q. Autophagy-mediated regulation of BACE1 protein trafficking and degradation. J Biol Chem. 2017;292:1679–90.
Du X, Huo X, Yang Y, Hu Z, Botchway BOA, Jiang Y, et al. miR-124 downregulates BACE 1 and alters autophagy in APP/PS1 transgenic mice. Toxicol Lett. 2017;280:195–205.
Adori M, Bhat S, Gramignoli R, Valladolid-Acebes I, Bengtsson T, Uhlèn M, et al. Hepatic innervations and nonalcoholic fatty liver disease. Semin Liver Dis. 2023;43:149–62.
Popov Y, Schuppan D. Targeting liver fibrosis: strategies for development and validation of antifibrotic therapies. Hepatology. 2009;50:1294–306.
Tashiro K, Satoh A, Utsumi T, Chung C, Iwakiri Y. Absence of Nogo-B (reticulon 4B) facilitates hepatic stellate cell apoptosis and diminishes hepatic fibrosis in mice. Am J Pathol. 2013;182:786–95.
Zheng Y, Lin J, Liu D, Wan G, Gu X, Ma J. Nogo-B promotes angiogenesis and improves cardiac repair after myocardial infarction via activating Notch1 signaling. Cell Death Dis. 2022;13:306.
Cervellati C, Valacchi G, Zuliani G. BACE1: from biomarker to Alzheimer’s disease therapeutical target. Aging. 2021;13:12299–300.
Bazzari FH, Bazzari AH. BACE1 inhibitors for Alzheimer’s disease: the past, present and any future? Molecules. 2022;27:8823.
Acknowledgements
Not applicable.
Funding
National Natural Science Foundation of China (No. 81570563).
Author information
Authors and Affiliations
Contributions
LG designed the research study. YingJie Zhuang and ZhengYi Liu performed the research. LiLi Gao and ZhengYi Liu provided help and advice. LiLi Gao and YingJie Zhuang analyzed the data. LiLi Gao wrote the manuscript. LiLi Gao reviewed and edited the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical approval
The animal experiment research protocol was approved by the Ethics Committee of The Second Medical Center and National Clinical Research Center for Geriatric Diseases, Chinese PLA General Hospital (202002BJ63) and performed in accordance with the “Guidelines for the care and use of experimental animals.”
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gao, L., Zhuang, Y. & Liu, Z. Reducing Nogo-B Improves Hepatic Fibrosis by Inhibiting BACe1-Mediated Autophagy. Tissue Eng Regen Med (2024). https://doi.org/10.1007/s13770-024-00641-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13770-024-00641-5