Abstract
Background:
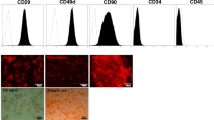
Diabetic neuropathy (DN) is the most common complication of diabetes, and approximately 50% of patients with this disease suffer from peripheral neuropathy. Nerve fiber loss in DN occurs due to myelin defects and is characterized by symptoms of impaired nerve function. Schwann cells (SCs) are the main support cells of the peripheral nervous system and play important roles in several pathways contributing to the pathogenesis and development of DN. We previously reported that human tonsil-derived mesenchymal stem cells differentiated into SCs (TMSC-SCs), named neuronal regeneration-promoting cells (NRPCs), which cells promoted nerve regeneration in animal models with peripheral nerve injury or hereditary peripheral neuropathy.
Methods:
In this study, NRPCs were injected into the thigh muscles of BKS-db/db mice, a commonly used type 2 diabetes model, and monitored for 26 weeks. Von Frey test, sensory nerve conduction study, and staining of sural nerve, hind foot pad, dorsal root ganglia (DRG) were performed after NRPCs treatment.
Results:
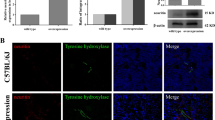
Von Frey test results showed that the NRPC treatment group (NRPC group) showed faster responses to less force than the vehicle group. Additionally, remyelination of sural nerve fibers also increased in the NRPC group. After NRPCs treatment, an improvement in response to external stimuli and pain sensation was expected through increased expression of PGP9.5 in the sole and TRPV1 in the DRG.
Conclusion:
The NRPCs treatment may alleviate DN through the remyelination and the recovery of sensory neurons, could provide a better life for patients suffering from complications of this disease.








Similar content being viewed by others
Data availability statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Williams R, Karuranga S, Malanda B, Saeedi P, Basit A, Besançon S, et al. Global and regional estimates and projections of diabetes-related health expenditure: results from the international diabetes federation diabetes atlas, 9th edition. Diabetes Research and Clinical Practice. 2020;162:108072.
Sempere-Bigorra M, Julián-Rochina I, Cauli O. Differences and similarities in neuropathy in type 1 and 2 diabetes: a systematic review. J Pers Med. 2021;11:230.
Allen MD, Choi IH, Kimpinski K, Doherty TJ, Rice CL. Motor unit loss and weakness in association with diabetic neuropathy in humans. Muscle Nerve. 2013;48:298–300.
Feldman EL, Callaghan BC, Pop-Busui R, Zochodne DW, Wright DE, Bennett DL, et al. Diabetic neuropathy. Nat Rev Dis Primers. 2019;5:41.
Zakin E, Abrams R, Simpson DM. Diabetic neuropathy. Semin Neurol. 2019;39:560–9.
Aziz N, Dash B, Wal P, Kumari P, Joshi P, Wal A. New horizons in diabetic neuropathies: an updated review on their pathology, diagnosis, mechanism, screening techniques, pharmacological, and future approaches. Curr Diabetes Rev. 2024;20:e201023222416.
Tesfaye S, Kempler P. Painful diabetic neuropathy. Diabetologia. 2005;48:805–7.
Eaton SE, Harris ND, Ibrahim S, Patel KA, Selmi F, Radatz M, et al. Increased sural nerve epineurial blood flow in human subjects with painful diabetic neuropathy. Diabetologia. 2003;46:934–9.
Gandhi RA, Marques JL, Selvarajah D, Emery CJ, Tesfaye S. Painful diabetic neuropathy is associated with greater autonomic dysfunction than painless diabetic neuropathy. Diabetes Care. 2010;33:1585–90.
Oyibo SO, Prasad YD, Jackson NJ, Jude EB, Boulton AJ. The relationship between blood glucose excursions and painful diabetic peripheral neuropathy: a pilot study. Diabet Med. 2002;19:870–3.
Selvarajah D, Wilkinson ID, Gandhi R, Griffiths PD, Tesfaye S. Microvascular perfusion abnormalities of the thalamus in painful but not painless diabetic polyneuropathy: a clue to the pathogenesis of pain in type 1 diabetes. Diabetes Care. 2011;34:718–20.
Sorensen L, Molyneaux L, Yue DK. The relationship among pain, sensory loss, and small nerve fibers in diabetes. Diabetes Care. 2006;29:883–7.
Schreiber AK, Nones CF, Reis RC, Chichorro JG, Cunha JM. Diabetic neuropathic pain: physiopathology and treatment. World J Diabetes. 2015;6:432–44.
Yorek M. Treatment for diabetic peripheral neuropathy: What have we learned from animal models? Curr Diabetes Rev. 2022;18:e040521193121.
Kobayashi M, Zochodne DW. Diabetic neuropathy and the sensory neuron: new aspects of pathogenesis and their treatment implications. J Diabetes Investig. 2018;9:1239–54.
Akkus G, Sert M. Diabetic foot ulcers: a devastating complication of diabetes mellitus continues non-stop in spite of new medical treatment modalities. World J Diabetes. 2022;13:1106–21.
Elafros MA, Kvalsund MP, Callaghan BC. The global burden of polyneuropathy-in need of an accurate assessment. JAMA Neurol. 2022;79:537–8.
Boulton AJM, Kempler P, Ametov A, Ziegler D. Whither pathogenetic treatments for diabetic polyneuropathy? Diabetes Metab Res Rev. 2013;29:327–33.
Akter S, Choubey M, Mohib MM, Arbee S, Sagor MAT, Mohiuddin MS. Stem cell therapy in diabetic polyneuropathy: recent advancements and future directions. Brain Sci. 2023;13:255.
Naruse K. Schwann cells as crucial players in diabetic neuropathy. In: Sango K, Yamauchi J, Ogata T, Susuki K, editors. Myelin: Basic and clinical advances. Singapore: Springer Singapore; 2019. p. 345–56.
Okawa T, Kamiya H, Himeno T, Kato J, Seino Y, Fujiya A, et al. Treatment of neural crest-like cells derived from induced pluripotent stem cells improves diabetic polyneuropathy in mice. Cell Transplant. 2013;22:1767–83.
Majd H, Amin S, Ghazizadeh Z, Cesiulis A, Arroyo E, Lankford K, Majd A, et al. Deriving Schwann cells from hPSCs enables disease modeling and drug discovery for diabetic peripheral neuropathy. Cell Stem Cell. 2023;30:632-47.e10.
De Gregorio C, Contador D, Díaz D, Cárcamo C, Santapau D, Lobos-Gonzalez L, et al. Human adipose-derived mesenchymal stem cell-conditioned medium ameliorates polyneuropathy and foot ulceration in diabetic BKS db/db mice. Stem Cell Res Ther. 2020;11:168.
Zhang Z, Liu Y, Zhou J. Neuritin promotes bone marrow-derived mesenchymal stem cell migration to treat diabetic peripheral neuropathy. Mol Neurobiol. 2022;59:6666–83.
Margiana R, Markov A, Zekiy AO, Hamza MU, Al-Dabbagh KA, Al-Zubaidi SH, et al. Clinical application of mesenchymal stem cell in regenerative medicine: a narrative review. Stem Cell Res Ther. 2022;13:366.
Abbaszadeh H, Ghorbani F, Derakhshani M, Movassaghpour AA, Yousefi M, Talebi M, Shamsasenjan K. Regenerative potential of wharton’s jelly-derived mesenchymal stem cells: a new horizon of stem cell therapy. J Cell Physiol. 2020;235:9230–40.
Ryu KH, Cho KA, Park HS, Kim JY, Woo SY, Jo I, et al. Tonsil-derived mesenchymal stromal cells: evaluation of biologic, immunologic and genetic factors for successful banking. Cytotherapy. 2012;14:1193–202.
Lee HJ, Kim YH, Choi DW, Cho KA, Park JW, Shin SJ, et al. Tonsil-derived mesenchymal stem cells enhance allogeneic bone marrow engraftment via collagen iv degradation. Stem Cell Res Ther. 2021;12:329.
Choi JS, Lee BJ, Park HY, Song JS, Shin SC, Lee JC, et al. Effects of donor age, long-term passage culture, and cryopreservation on tonsil-derived mesenchymal stem cells. Cell Physiol Biochem. 2015;36:85–99.
Oh SY, Choi YM, Kim HY, Park YS, Jung SC, Park JW, et al. Application of tonsil-derived mesenchymal stem cells in tissue regeneration: concise review. Stem Cells. 2019;37:1252–60.
Jung N, Park S, Choi Y, Park JW, Hong YB, Park HH, et al. Tonsil-derived mesenchymal stem cells differentiate into a schwann cell phenotype and promote peripheral nerve regeneration. Int J Mol Sci. 2016;17:1867.
Park S, Jung N, Myung S, Choi Y, Chung KW, Choi BO, Jung SC. Differentiation of human tonsil-derived mesenchymal stem cells into schwann-like cells improves neuromuscular function in a mouse model of charcot-marie-tooth disease type 1a. Int J Mol Sci. 2018;19:2393.
Bosch-Queralt M, Fledrich R, Stassart RM. Schwann cell functions in peripheral nerve development and repair. Neurobiol Dis. 2023;176:105952.
Li J, Guan R, Pan L. Mechanism of schwann cells in diabetic peripheral neuropathy: a review. Medicine (Baltimore). 2023;102:e32653.
Nam YH, Park S, Yum Y, Jeong S, Park HE, Kim HJ, et al. Preclinical efficacy of peripheral nerve regeneration by schwann cell-like cells differentiated from human tonsil-derived mesenchymal stem cells in c22 mice. Biomedicines. 2023;11:3334.
De Gregorio C, Contador D, Campero M, Ezquer M, Ezquer F. Characterization of diabetic neuropathy progression in a mouse model of type 2 diabetes mellitus. Biol Open. 2018;7:bio036830.
Shi TJ, Zhang MD, Zeberg H, Nilsson J, Grünler J, Liu SX, et al. Coenzyme q10 prevents peripheral neuropathy and attenuates neuron loss in the db−/db− mouse, a type 2 diabetes model. Proc Natl Acad Sci U S A. 2013;110:690–5.
Lee SM, Bressler R. Prevention of diabetic nephropathy by diet control in the db/db mouse. Diabetes. 1981;30:106–11.
Wald C, Wu C. Of mice and women: the bias in animal models. Science. 2010;327:1571–2.
Garbay B, Heape AM, Sargueil F, Cassagne C. Myelin synthesis in the peripheral nervous system. Prog Neurobiol. 2000;61:267–304.
Lauria G, Cornblath DR, Johansson O, McArthur JC, Mellgren SI, Nolano M, et al. Efns guidelines on the use of skin biopsy in the diagnosis of peripheral neuropathy. Eur J Neurol. 2005;12:747–58.
Hu Q, Wang Q, Wang C, Tai Y, Liu B, Shao X, et al. Trpv1 channel contributes to the behavioral hypersensitivity in a rat model of complex regional pain syndrome type 1. Front Pharmacol. 2019;10:453.
Szabadfi K, Pinter E, Reglodi D, Gabriel R. Chapter one - neuropeptides, trophic factors, and other substances providing morphofunctional and metabolic protection in experimental models of diabetic retinopathy. In: Jeon KW, editor. International review of cell and molecular biology. 311: Academic Press; 2014. p. 1–121.
Kan M, Guo G, Singh B, Singh V, Zochodne DW. Glucagon-like peptide 1, insulin, sensory neurons, and diabetic neuropathy. J Neuropathol Exp Neurol. 2012;71:494–510.
Sullivan KA, Hayes JM, Wiggin TD, Backus C, Su OhS, Lentz SI, et al. Mouse models of diabetic neuropathy. Neurobiol Dis. 2007;28:276–85.
Wright DE, Johnson MS, Arnett MG, Smittkamp SE, Ryals JM. Selective changes in nocifensive behavior despite normal cutaneous axon innervation in leptin receptor-null mutant (db/db) mice. J Peripher Nerv Syst. 2007;12:250–61.
Norido F, Canella R, Zanoni R, Gorio A. Development of diabetic neuropathy in the c57bl/ks (db/db) mouse and its treatment with gangliosides. Exp Neurol. 1984;83:221–32.
Sima AAF, Robertson DM. Peripheral neuropathy in mutant diabetic mouse [c57bl/ks (db/db)]. Acta Neuropathol. 1978;41:85–9.
Moore SA, Peterson RG, Felten DL, Cartwright TR, O’Connor BL. Reduced sensory and motor conduction velocity in 25-week-old diabetic [c57blks (dbdb)] mice. Exp Neurol. 1980;70:548–55.
Sumner CJ, Sheth S, Griffin JW, Cornblath DR, Polydefkis M. The spectrum of neuropathy in diabetes and impaired glucose tolerance. Neurology. 2003;60:108–11.
Tavee J, Zhou L. Small fiber neuropathy: a burning problem. Cleve Clin J Med. 2009;76:297–305.
Fan B, Li C, Szalad A, Wang L, Pan W, Zhang R, et al. Mesenchymal stromal cell-derived exosomes ameliorate peripheral neuropathy in a mouse model of diabetes. Diabetologia. 2020;63:431–43.
Lee DK. Basic skills in nerve conduction studies. Ann Clin Neurophysiol. 1999;1:202–9.
Ko KR, Lee J, Lee D, Nho B, Kim S. Hepatocyte growth factor (hgf) promotes peripheral nerve regeneration by activating repair schwann cells. Sci Rep. 2018;8:8316.
Maina F, Hilton MC, Ponzetto C, Davies AM, Klein R. Met receptor signaling is required for sensory nerve development and HGF promotes axonal growth and survival of sensory neurons. Genes Dev. 1997;11:3341–50.
Kessler JA, Shaibani A, Sang CN, Christiansen M, Kudrow D, Vinik A, et al. Gene therapy for diabetic peripheral neuropathy: a randomized, placebo-controlled phase III study of VM202, a plasmid DNA encoding human hepatocyte growth factor. Clin Transl Sci. 2021;14:1176–84.
Pabbidi RM, Yu S-Q, Peng S, Khardori R, Pauza ME, Premkumar LS. Influence of trpv1 on diabetes-induced alterations in thermal pain sensitivity. Mol Pain. 2008;4:9.
Dauch JR, Yanik BM, Hsieh W, Oh SS, Cheng HT. Neuron-astrocyte signaling network in spinal cord dorsal horn mediates painful neuropathy of type 2 diabetes. Glia. 2012;60:1301–15.
Acknowledgements
This study was supported by a Korean Fund for Regenerative Medicine (KFRM) grant, funded by the Korea government (the Ministry of Science and ICT, the Ministry of Health & Welfare) (22C0627L1-11), a Basic Science Research Program through the NRF funded by the Ministry of Education (2022R1I1A01064295) and the RP-Grant 2023 of Ewha Womans University.
Author information
Authors and Affiliations
Contributions
S-CJ conceived and designed the study; YY, SP, YHN, JY and SH performed experiments; YY, SP and S-CJ analyzed the data; HJK and JL resources; YY, SP and S-CJ wrote the paper All authors have read and agreed to the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
H.J.K. and J.L. are employees of Cellatoz Therapeutics, Inc.; S.-C.J. is a scientific advisory board member of Cellatoz Therapeutics, Inc.; The other authors declare no conflict of interest.
Ethical statement
The study protocol was approved by the Ewha Womans University Medical Center (EWUMC) institutional review board (IRB number: EUMC-2021-09-036). All the experimental procedures were reviewed and approved by the ethics committee for animal research at Ewha Woman’s University (EWHA MEDIACUC 22-005-3). Informed Consent Statement: Informed written consent was obtained from all the patients participating in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yum, Y., Park, S., Nam, Y.H. et al. Therapeutic Effect of Schwann Cell-Like Cells Differentiated from Human Tonsil-Derived Mesenchymal Stem Cells on Diabetic Neuropathy in db/db Mice. Tissue Eng Regen Med (2024). https://doi.org/10.1007/s13770-024-00638-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13770-024-00638-0