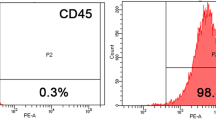
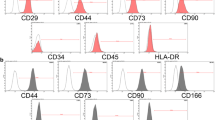
Abstract
Background:
Temporomandibular joint osteoarthritis (TMJOA) is a degenerative disease affecting the cartilage and subchondral bone, leading to temporomandibular joint pain and dysfunction. The complex nature of TMJOA warrants effective alternative treatments, and mesenchymal stem cells (MSCs) have shown promise in regenerative therapies. The aim of this study is twofold: firstly, to ascertain the optimal interferon-gamma (IFN-γ)-primed MSC cell line for TMJOA treatment, and secondly, to comprehensively evaluate the therapeutic efficacy of IFN-γ-primed mesenchymal stem cells derived from the human umbilical cord matrix in a rat model of TMJOA.
Methods:
We analyzed changes in the expression of several key genes associated with OA protection in MSC-secreted compounds. Following this, we performed co-culture experiments using a transwell system to predict gene expression changes in primed MSCs in the TMJOA environment. Subsequently, we investigated the efficacy of the selected IFN-γ-primed human umbilical cord matrix-derived MSCs (hUCM-MSCs) for TMJOA treatment in a rat model.
Results:
IFN-γ-primed MSCs exhibited enhanced expression of IDO, TSG-6, and FGF-2. Moreover, co-culturing with rat OA chondrocytes induced a decrease in pro-inflammatory and extracellular matrix degradation factors. In the rat TMJOA model, IFN-γ-primed MSCs with elevated IDO1, TSG-6, and FGF2 expression exhibited robust anti-inflammatory and therapeutic capacities, promoting the improvement of the inflammatory environment and cartilage regeneration.
Conclusion:
These findings underscore the importance of prioritizing the mitigation of the inflammatory milieu in TMJOA treatment and highlight IFN-γ-primed MSCs secreting these three factors as a promising, comprehensive therapeutic strategy.





Similar content being viewed by others
Data availability statements
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Zhao Y, Xie L. An update on mesenchymal stem cell-centered therapies in temporomandibular joint osteoarthritis. Stem Cells Int. 2021;2021:6619527.
Ying W, Yuan F, He P, Ji P. Inhibition of Notch1 protects against IL-1β-induced inflammation and cartilage destruction in tempromandibular chondrocytes. Mol Med Rep. 2017;15:4391–7.
Zarb GA, Carlsson GE. Temporomandibular disorders: osteoarthritis. J Orofac pain. 1999;13:295–306.
Wang XD, Zhang JN, Gan YH, Zhou YH. Current understanding of pathogenesis and treatment of TMJ osteoarthritis. J Dent Res. 2015;94:666–73.
Li B, Guan G, Mei L, Jiao K, Li H. Pathological mechanism of chondrocytes and the surrounding environment during osteoarthritis of temporomandibular joint. J Cell Mol Med. 2021;25:4902–11.
Wu CL, Harasymowicz NS, Klimak MA, Collins KH, Guilak F. The role of macrophages in osteoarthritis and cartilage repair. Osteoarthr Cartil. 2020;28:544–54.
Lu K, Ma F, Yi D, Yu H, Tong L, Chen D. Molecular signaling in temporomandibular joint osteoarthritis. J Orthop Translat. 2021;32:21–7.
Ahmad N, Ansari MY, Haqqi TM. Role of iNOS in osteoarthritis: pathological and therapeutic aspects. J Cell Physiol. 2020;235:6366–76.
Nurul AA, Azlan M, Ahmad Mohd Zain MR, Sebastian AA, Fan YZ, Fauzi MB. Mesenchymal stem cells: current concepts in the management of inflammation in osteoarthritis. Biomedicines. 2021;9:785.
Tanaka E, Detamore MS, Mercuri LG. Degenerative disorders of the temporomandibular joint: etiology, diagnosis, and treatment. J Dent Res. 2008;87:296–307.
Cardoneanu A, Macovei LA, Burlui AM, Mihai IR, Bratoiu I, Rezus II, et al. Temporomandibular joint osteoarthritis: pathogenic mechanisms involving the cartilage and subchondral bone, and potential therapeutic strategies for joint regeneration. Int J Mol Sci. 2022;24:171.
Pagotto LEC, Santos TS, Pastore GP. The efficacy of mesenchymal stem cells in regenerating structures associated with the temporomandibular joint: a systematic review. Arch Oral Biol. 2021;125:105104.
Kim H, Lee BK. Anti-inflammatory effect of adipose-derived stromal vascular fraction on osteoarthritis temporomandibular joint synoviocytes. Tissue Eng Regen Med. 2020;17:351–62.
Kim H, Yang G, Park J, Choi J, Kang E, Lee BK. Therapeutic effect of mesenchymal stem cells derived from human umbilical cord in rabbit temporomandibular joint model of osteoarthritis. Sci Rep. 2019;9:13854.
Kim JY, Rhim WK, Cha SG, Woo J, Lee JY, Park CG, et al. Bolstering the secretion and bioactivities of umbilical cord MSC-derived extracellular vesicles with 3D culture and priming in chemically defined medial. Nano Converg. 2022;9:57.
Guerrouahen BS, Sidahmed H, Sulaiti A, AIkhulaifi M, Cugno C. Enhancing mesenchymal stromal cell immunomodulation for treating conditions influenced by the immune system. Stem Cells Int. 2019;2019:7219297.
Kim DS, Jang IK, Lee MW, Ko YJ, Lee DH, Lee JW, et al. Enhanced immunosuppressive properties of human mesenchymal stem cells primed by interferon-γ. EBioMedicine. 2018;28:261–73.
Duijvestein M, Wildenberg ME, Welling MM, Hennink S, Molendijk I, van Zuylen VL, et al. Pretreatment with interferon-γ enhances the therapeutic activity of mesenchymal stromal cells in animal models of colitis. Stem Cells. 2011;29:549–58.
Kanai R, Nakashima A, Doi S, Kimura T, Yoshida K, Maeda S, et al. Interferon-γ enhances the therapeutic effect of mesenchymal stem cells on experimental renal fibrosis. Sci Rep. 2021;11:850.
Mitchell KE, Weiss ML, Mitchell BM, Martin P, Davis D, Morales L, et al. Matrix cells from Wharton’s jelly form neurons and glia. Stem Cells. 2003;21:50–60.
Embree MC, Chen M, Pylawka S, Kong D, Iwaoka GM, Kalajzic I, et al. Exploiting endogenous fibrocartilage stem cells to regenerate cartilage and repair joint injury. Nat Commun. 2016;7:13073.
Zhou PH, Liu SQ, Peng H. The effect of hyaluronic acid on IL-1ß-induced chondrocyte apoptosis in a rat model of osteoarthritis. J Orthop Res. 2008;7:1643–8.
Yun SY, Kim Y, Kim H, Lee BK. Effective technical protocol for producting a mono-iodoacetate- induced temporomandibular joint osteoarthritis in a rat model. Tissue Eng Part C Methods. 2023;29:438–45.
Ren K. An improved method for assessing mechanical allodynia in the rat. Physiol Behav. 1999;67:711–6.
Shen P, Jiao Z, Zheng JS, Xu WF, Zhang SY, Qin A, et al. Injecting vascular endothelial growth factor into the temporomandibular joint induces osteoarthritis in mice. Sci Rep. 2015;5:16244.
Mancuso P, Raman S, Glynn A, Barry F, Murphy JM. Mesenchymal stem cell therapy for osteoarthritis: the critical role of the cell secretome. Front Bioeng Biotechnol. 2019;7:9.
Harrell CR, Markovic BS, Fellabaum C, Arsenijevic A, Volarevic V. Mesenchymal stem cell-based therapy of osteoarthritis: current knowledge and future perspectives. Biomed Pharmacother. 2019;109:2318–26.
Madrigal M, Rao KS, Riordan NH. A review of therapeutic effects of mesenchymal stem cell secretions and induction of secretory modification by different culture methods. J Transl Med. 2014;12:260.
Jeong SY, Kim DH, Ha J, Jin HJ, Kwon SJ, Chang JW, et al. Thrombospondin-2 secreted by human umbilical cord blood-derived mesenchymal stem cells promotes chondrogenic differentiation. Stem Cells. 2013;1:2136–48.
Jeon HJ, Yoon KA, An ES, Kang TW, Sim YB, Ahn J, et al. Therapeutic effects of human umbilical cord blood-derived mesenchymal stem cells combined with cartilage acellular matrix mediated via bone morphogenic protein 6 in a rabbit model of articular cruciate ligament transection. Stem Cell Rev Rep. 2020;16:596–611.
Munn DH, Mellor AL. IDO in the tumor microenvironment: inflammation, counter-regulation, and tolerance. Trends Immunol. 2016;37:193–207.
Li R, Wei F, Yu J, Ren X, Hao X. IDO inhibits T-cell function through suppressing Vav1 expression and activation. Cancer Biol Ther. 2009;8:1402–8.
Heidar F, Razmkhah M, Razban V, Erfani N. Effects of indoleamine 2,3-dioxygenase (IDO) silencing on immunomodulatory function and cancer-promoting characteristic of adipose-derived mesenchymal stem cells (ASCs). Cell Biol Int. 2021;45:2544–56.
François M, Mourez RR, Li M, Galipeau J. Human MSC suppression correlates with cytokine induction of indolamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Mol Ther. 2012;20:187–95.
Fernandes TL, Gomoll AH, Lattermann C, Hernandez AJ, Bueno DF, Amano MT. Macrophage: a potential target on cartilage regeneration. Front Immunol. 2020;11:111.
Hu Y, Gui Z, Zhou Y, Xia L, Lin K, Xu Y. Quercein alleviates rat osteoarthritis by inhibiting inflammation and apoptosis of chondrocytes, modulating synovial macrophages polarization to M2 macrophages. Free Radic Biol Med. 2019;145:146–60.
Chou CH, Attarian DE, Wisniewski HG, Band PA, Kraus VB. TSG-6- a double-edged sword for osteoarthritis (OA). Osteoarthritis Cartilage. 2018;26:245–54.
Zhang S, Fang J, Liu Z, Hou P, Cao L, Zhang Y, et al. Inflammatory cytokines-stimulated human muscle stem cells ameliorate ulcerative colitis via the IDO-TSG axis. Stem Cell Res Ther. 2021;12:50.
Wang G, Cao K, Liu K, Xue Y, Roberts A, Li F, et al. Kynurenic acid, an IDO metabolite, controls TSG-6-mediated immunosuppression of human mesenchymal stem cells. Cell Death Differ. 2018;25:1209–23.
Fredriksson L, Alstergren P, Kopp S. Tumor necrosis factor-alpha in temporomandibular joint synovial fluid predicts treatment effects on pain by intra-articular glucocorticoid treatment. ediators Inflamm. 2006;2006:59425.
Fang T, Zhou X, Jin M, Nie J, Li X. Molecular mechanisms of mechanical load-iduced osteoarthritis. Int Orthop. 2021;45:1125–36.
Agarwal S, Long P, Gassner R, Piesco NP, Buckley MJ. Cyclic tensile strain suppresses catabolic effects of interleukin-1beta in fibrochondrocytes from the temporomandibular joint. Arthritis Rheum. 2001;44:608–17.
Okamura G, Ebina K, Hirao M, Chijimatsu R, Yonetani Y, Etani Y, et al. Promoting effect of basic fibroblast growth factor in synovial mesenchymal stem cell-based cartilage regeneration. Int J Mol Sci. 2021;22:300.
Zaragosi LE, Ailhaud G, Dani C. Autocrine fibroblast growth factor 2 signaling is critical for self-renewal of human multipotent adipose-derived stem cells. Stem Cells. 2006;24:2412–9.
Chia SL, Sawaji Y, Burleigh A, McLean C, Inglis J, Saklatvala J, et al. Fibroblast growth factor 2 is an intrinsic chondroprotective agent that suppresses ADAMTS-5 and delay cartilage degradation in murine osteoarthritis. Arthritis Rheum. 2009;60:2019–27.
Jeong SY, Ha J, Lee M, Jin HJ, Kim DH, Choi SJ, et al. Autocrine action of thrombospondin-2 determines the chondrogenic differentiation potential and suppresses hypertrophic maturation of human umbilical cord blood-derived mesenchymal stem cells. Stem Cells. 2015;22:3291–303.
Ye F, Xu H, Yin H, Zhao X, Li D, Zhu Q, et al. The role of BMP6 in the proliferation and differentiation of chicken cartilage cells. PLoS One. 2019;14:e0204384.
Acknowledgements
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: H21C0168).
Author information
Authors and Affiliations
Contributions
HK was responsible for drafting the manuscript, analyzing and interpreting the data, as well as conducting and designing this study. YK and SYY contributed to the execution of the study. BKL is the corresponding author, responsible for the conception and critical revision of the article.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no financial conflicts of interest.
Ethical statement
This work was approved by the Public Institutional Bioethics Committee designated by the MOHW (IRB no. 2020-1825-011). All animal experiments were performed in accordance with relevant guidelines and regulations of the Institutional Animal Care and Use Committee of Asan Medical Center (authorization no. 2021-12-208), which is accredited for laboratory animal care by the Ministry of Food and Drug Safety of South Korea.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, H., Kim, Y., Yun, SY. et al. Efficacy of IFN-γ-Primed Umbilical Cord-Derived Mesenchymal Stem Cells on Temporomandibular Joint Osteoarthritis. Tissue Eng Regen Med 21, 473–486 (2024). https://doi.org/10.1007/s13770-023-00620-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-023-00620-2