Abstract
Background:
Mesenchymal stem cells (MSCs) have been highlighted as a potent therapeutic option for conditions with excessive osteoclast activity such as systemic and local bone loss in rheumatic disease. In addition to their immunomodulatory functions, MSCs also directly suppress osteoclast differentiation and activation by secreting osteoprotegerin (OPG) and IL-10 but the underlying mechanisms are still to be clarified. Tumor necrosis factor-stimulated gene-6 (TSG-6) is a potent anti-inflammatory molecule that inhibits osteoclast activation and has been shown to mediate MSC’s immunomodulatory functions. In this study, we aimed to determine whether adipose tissue-derived MSC (ADMSC) inhibits the differentiation from osteoclast precursors to mature osteoclasts through TSG-6.
Methods:
Human ADMSCs were co-cultured with bone marrow-derived monocyte/macrophage (BMMs) from DBA/1J or B6 mouse in the presence of osteoclastogenic condition (M-CSF 10 ng/mL and RANKL 10 ng/mL). In some co-culture groups, ADMSCs were transfected with siRNA targeting TSG-6 or OPG to determine their role in osteoclastogenesis. Tartrate-resistant acid phosphatase (TRAP) activity in culture supernatant and mRNA expression of osteoclast markers were investigated. TRAP+ multinucleated cells and F-actin ring formation were counted.
Results:
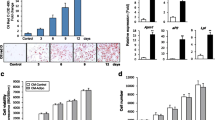
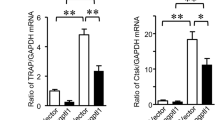
ADMSCs significantly inhibited osteoclast differentiation under osteoclastogenic conditions. Suppression of TSG-6 significantly reversed the inhibition of osteoclast differentiation in a degree similar to that of OPG based on TRAP activity, mRNA expression of osteoclast markers, and numbers of TRAP+ multinucleated cell and F-actin ring formation.
Conclusion:
This study demonstrated that ADMSCs inhibit osteoclast differentiation through TSG-6 under osteoclastogenic conditions.





Similar content being viewed by others
Data availability statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Adami G, Fassio A, Rossini M, Caimmi C, Giollo A, Orsolini G, et al. Osteoporosis in rheumatic diseases. Int J Mol Sci. 2019;20:5867.
van Staa TP, Geusens P, Bijlsma JW, Leufkens HG, Cooper C. Clinical assessment of the long-term risk of fracture in patients with rheumatoid arthritis. Arthritis Rheum. 2006;54:3104–12.
Liu L, Webster TJ. In situ sensor advancements for osteoporosis prevention, diagnosis, and treatment. Curr Osteoporos Rep. 2016;14:386–95.
Cheng C, Wentworth K, Shoback DM. New frontiers in osteoporosis therapy. Annu Rev Med. 2020;71:277–88.
Ichioka N, Inaba M, Kushida T, Esumi T, Takahara K, Inaba K, et al. Prevention of senile osteoporosis in SAMP6 mice by intrabone marrow injection of allogeneic bone marrow cells. Stem Cells. 2002;20:542–51.
Ocarino Nde M, Boeloni JN, Jorgetti V, Gomes DA, Goes AM, Serakides R. Intra-bone marrow injection of mesenchymal stem cells improves the femur bone mass of osteoporotic female rats. Connect Tissue Res. 2010;51:426–33.
Kiernan J, Hu S, Grynpas MD, Davies JE, Stanford WL. Systemic mesenchymal stromal cell transplantation prevents functional bone loss in a mouse model of age-related osteoporosis. Stem Cells Transl Med. 2016;5:683–93.
Ye X, Zhang P, Xue S, Xu Y, Tan J, Liu G. Adipose-derived stem cells alleviate osteoporosis by enhancing osteogenesis and inhibiting adipogenesis in a rabbit model. Cytotherapy. 2014;16:1643–55.
Takano T, Li YJ, Kukita A, Yamaza T, Ayukawa Y, Moriyama K, et al. Mesenchymal stem cells markedly suppress inflammatory bone destruction in rats with adjuvant-induced arthritis. Lab Invest. 2014;94:286–96.
Abe T, Sumi K, Kunimatsu R, Oki N, Tsuka Y, Nakajima K, et al. The effect of mesenchymal stem cells on osteoclast precursor cell differentiation. J Oral Sci. 2019;61:30–5.
Day AJ, Milner CM. TSG-6: a multifunctional protein with anti-inflammatory and tissue-protective properties. Matrix Biol. 2019;78–79:60–83.
Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63.
Usunier B, Brossard C, L’Homme B, Linard C, Benderitter M, Milliat F, et al. HGF and TSG-6 released by mesenchymal stem cells attenuate colon radiation-induced fibrosis. Int J Mol Sci. 2021;22:1790.
Ding Y, Gong P, Jiang J, Feng C, Li Y, Su X, et al. Mesenchymal stem/stromal cells primed by inflammatory cytokines alleviate psoriasis-like inflammation via the TSG-6-neutrophil axis. Cell Death Dis. 2022;13:996.
Yang S, Liang X, Song J, Li C, Liu A, Luo Y, et al. A novel therapeutic approach for inflammatory bowel disease by exosomes derived from human umbilical cord mesenchymal stem cells to repair intestinal barrier via TSG-6. Stem Cell Res Ther. 2021;12:315.
Mahoney DJ, Swales C, Athanasou NA, Bombardieri M, Pitzalis C, Kliskey K, et al. TSG-6 inhibits osteoclast activity via an autocrine mechanism and is functionally synergistic with osteoprotegerin. Arthritis Rheum. 2011;63:1034–43.
Aubin JE, Bonnelye E. Osteoprotegerin and its ligand: a new paradigm for regulation of osteoclastogenesis and bone resorption. Osteoporos Int. 2000;11:905–13.
Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141–50.
Sudres M, Norol F, Trenado A, Grégoire S, Charlotte F, Levacher B, et al. Bone marrow mesenchymal stem cells suppress lymphocyte proliferation in vitro but fail to prevent graft-versus-host Disease in mice. J Immunol. 2006;176:7761–7.
Zhu H, Jiang XX, Guo ZK, Li H, Su YF, Yao HY, et al. Tumor necrosis factor-alpha alters the modulatory effects of mesenchymal stem cells on osteoclast formation and function. Stem Cells Dev. 2009;18:1473–84.
Milner CM, Tongsoongnoen W, Rugg MS, Day AJ. The molecular basis of inter-alpha-inhibitor heavy chain transfer on to hyaluronan. Biochem Soc Trans. 2007;35:672–6.
Mbalaviele G, Jaiswal N, Meng A, Cheng L, Van Den Bos C, Thiede M. Human mesenchymal stem cells promote human osteoclast differentiation from CD34 + bone marrow hematopoietic progenitors. Endocrinology. 1999;140:3736–43.
Oshita K, Yamaoka K, Udagawa N, Fukuyo S, Sonomoto K, Maeshima K, et al. Human mesenchymal stem cells inhibit osteoclastogenesis through osteoprotegerin production. Arthritis Rheum. 2011;63:1658–67.
Lee TH, Wisniewski HG, Vilcek J. A novel secretory Tumor necrosis factor-inducible protein (TSG-6) is a member of the family of hyaluronate binding proteins, closely related to the adhesion receptor CD44. J Cell Biol. 1992;116:545–57.
Tsukahara S, Ikeda R, Goto S, Yoshida K, Mitsumori R, Sakamoto Y, et al. Tumour necrosis factor alpha-stimulated gene-6 inhibits osteoblastic differentiation of human mesenchymal stem cells induced by osteogenic differentiation medium and BMP-2. Biochem J. 2006;398:595–603.
Mahoney DJ, Mikecz K, Ali T, Mabilleau G, Benayahu D, Plaas A, et al. TSG-6 regulates bone remodeling through inhibition of osteoblastogenesis and osteoclast activation. J Biol Chem. 2008;283:25952–62.
de Sousa Abreu R, Penalva LO, Marcotte EM, Vogel C. Global signatures of protein and mRNA expression levels. Mol Biosyst. 2009;5:1512–26.
Maier T, Güell M, Serrano L. Correlation of mRNA and protein in complex biological samples. FEBS Lett. 2009;583:3966–73.
Acknowledgements
This study was supported by Dongguk University Research Fund of 2022, Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2018R1D1A1B07050276), Severance Hospital,Yonsei University Health System (4-2021-0599) and the Korean Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health and Welfare, Republic of Korea (HI14C1324).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest.
Ethical statement
All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of Yonsei University Health System (approval number: 2020 − 0291).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary figure 1
Efficacy of siRNA transfection (TSG-6). ADMSCs were transfected with siRNA targeting TSG-6 according to manufacturer’s instructions. Compared to the ADMSCS of no siRNA transfection (No transf.) and scramble siRNA transfection (Scr. siRNA), TSG-6 mRNA suppression was apparent in ADMSCs transfected with siRNA targeting TSG-6 as early as 10 hours after transfection and was maintained through 24 hours after (JPG 42.7 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, K., Ko, E. & Park, Y. Adipose Tissue-Derived Mesenchymal Stem Cell Inhibits Osteoclast Differentiation through Tumor Necrosis Factor Stimulated Gene-6. Tissue Eng Regen Med 21, 587–594 (2024). https://doi.org/10.1007/s13770-023-00619-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-023-00619-9