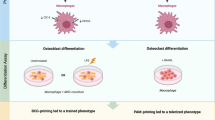
Abstract
Background:
Human lymph node (HuLN) models have emerged with invaluable potential for immunological research and therapeutic application given their fundamental role in human health and disease. While fibroblastic reticular cells (FRCs) are instrumental to HuLN functioning, their inclusion and recognition of importance for organotypic in vitro lymphoid models remain limited.
Methods:
Here, we established an in vitro three-dimensional (3D) model in a collagen-fibrin hydrogel with primary FRCs and a dendritic cell (DC) cell line (MUTZ-3 DC). To study and characterise the cellular interactions seen in this 3D FRC-DC organotypic model compared to the native HuLN; flow cytometry, immunohistochemistry, immunofluorescence and cytokine/chemokine analysis were performed.
Results:
FRCs were pivotal for survival, proliferation and localisation of MUTZ-3 DCs. Additionally, we found that CD1a expression was absent on MUTZ-3 DCs that developed in the presence of FRCs during cytokine-induced MUTZ-3 DC differentiation, which was also seen with primary monocyte-derived DCs (moDCs). This phenotype resembled HuLN-resident DCs, which we detected in primary HuLNs, and these CD1a− MUTZ-3 DCs induced T cell proliferation within a mixed leukocyte reaction (MLR), indicating a functional DC status. FRCs expressed podoplanin (PDPN), CD90 (Thy-1), CD146 (MCAM) and Gremlin-1, thereby resembling the DC supporting stromal cell subset identified in HuLNs.
Conclusion:
This 3D FRC-DC organotypic model highlights the influence and importance of FRCs for DC functioning in a more realistic HuLN microenvironment. As such, this work provides a starting point for the development of an in vitro HuLN.





Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Roozendaal R, Mebius RE, Kraal G. The conduit system of the lymph node. Int Immunol. 2008;20:1483–7.
Krishnamurty AT, Turley SJ. Lymph node stromal cells: cartographers of the immune system. Nat Immunol. 2020;21:369–80.
Bajenoff M, Egen JG, Koo LY, Laugier JP, Brau F, Glaichenhaus N, Germain RN. Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity. 2006;25:989–1001.
Roozendaal R, Mebius RE. Stromal cell-immune cell interactions. Annu Rev Immunol. 2011;29:23–43.
Rodda LB, Lu E, Bennett ML, Sokol CL, Wang X, Luther SA, et al. Single-cell rna sequencing of lymph node stromal cells reveals niche-associated heterogeneity. Immunity. 2018;48:1014e6-28e6.
Grasso C, Pierie C, Mebius RE, van Baarsen LGM. Lymph node stromal cells: Subsets and functions in health and disease. Trends Immunol. 2021;42:920–36.
Acton SE, Astarita JL, Malhotra D, Lukacs-Kornek V, Franz B, Hess PR, et al. Podoplanin-rich stromal networks induce dendritic cell motility via activation of the c-type lectin receptor clec-2. Immunity. 2012;37:276–89.
Girard JP, Moussion C, Forster R. Hevs, lymphatics and homeostatic immune cell trafficking in lymph nodes. Nat Rev Immunol. 2012;12:762–73.
Acton SE, Onder L, Novkovic M, Martinez VG, Ludewig B. Communication, construction, and fluid control: lymphoid organ fibroblastic reticular cell and conduit networks. Trends Immunol. 2021;42:782–94.
Collin M, Bigley V. Human dendritic cell subsets: An update. Immunology. 2018;154:3–20.
Gerner MY, Kastenmuller W, Ifrim I, Kabat J, Germain RN. Histo-cytometry: a method for highly multiplex quantitative tissue imaging analysis applied to dendritic cell subset microanatomy in lymph nodes. Immunity. 2012;37:364–76.
Gerner MY, Casey KA, Kastenmuller W, Germain RN. Dendritic cell and antigen dispersal landscapes regulate t cell immunity. J Exp Med. 2017;214:3105–22.
Baptista AP, Gola A, Huang Y, Milanez-Almeida P, Torabi-Parizi P, Urban JF Jr, et al. The chemoattractant receptor ebi2 drives intranodal naive cd4(+) t cell peripheralization to promote effective adaptive immunity. Immunity. 2019;50:1188e6-201e6.
van de Ven R, van den Hout MF, Lindenberg JJ, Sluijter BJ, van Leeuwen PA, Lougheed SM, et al. Characterization of four conventional dendritic cell subsets in human skin-draining lymph nodes in relation to t-cell activation. Blood. 2011;118:2502–10.
Segura E, Soumelis V. Of human dc migrants and residents. Immunity. 2017;46:342–4.
Novkovic M, Onder L, Cupovic J, Abe J, Bomze D, Cremasco V, et al. Topological small-world organization of the fibroblastic reticular cell network determines lymph node functionality. PLoS Biol. 2016;14:e1002515.
Kapoor VN, Muller S, Keerthivasan S, Brown M, Chalouni C, Storm EE, et al. Gremlin 1(+) fibroblastic niche maintains dendritic cell homeostasis in lymphoid tissues. Nat Immunol. 2021;22:571–85.
Ozulumba T, Montalbine AN, Ortiz-Cardenas JE, Pompano RR. New tools for immunologists: models of lymph node function from cells to tissues. Front Immunol. 2023;14:1183286.
Goyal G, Prabhala P, Mahajan G, Bausk B, Gilboa T, Xie L, et al. Ectopic lymphoid follicle formation and human seasonal influenza vaccination responses recapitulated in an organ-on-a-chip. Adv Sci (Weinh). 2022;9:e2103241.
Wagar LE, Salahudeen A, Constantz CM, Wendel BS, Lyons MM, Mallajosyula V, et al. Modeling human adaptive immune responses with tonsil organoids. Nat Med. 2021;27:125–35.
Belanger MC, Ball AG, Catterton MA, Kinman AWL, Anbaei P, Groff BD, et al. Acute lymph node slices are a functional model system to study immunity ex vivo. ACS Pharmacol Transl Sci. 2021;4:128–42.
Knoblich K, Cruz Migoni S, Siew SM, Jinks E, Kaul B, Jeffery HC, et al. The human lymph node microenvironment unilaterally regulates t-cell activation and differentiation. PLoS Biol. 2018;16:e2005046.
Braham MVJ, van Binnendijk RS, Buisman AM, Mebius RE, de Wit J, van Els C. A synthetic human 3d in vitro lymphoid model enhancing b-cell survival and functional differentiation. iScience. 2023;26:105741.
Kwee BJ, Akue A, Sung KE. On-chip human lymph node stromal network for evaluating dendritic cell and t-cell trafficking. BIORXIV. 2023;03:533042.
Kosten IJ, Spiekstra SW, de Gruijl TD, Gibbs S. Mutz-3 derived langerhans cells in human skin equivalents show differential migration and phenotypic plasticity after allergen or irritant exposure. Toxicol Appl Pharmacol. 2015;287:35–42.
Koning JJ, Rodrigues Neves CT, Schimek K, Thon M, Spiekstra SW, Waaijman T, et al. A multi-organ-on-chip approach to investigate how oral exposure to metals can cause systemic toxicity leading to langerhans cell activation in skin. Front Toxicol. 2021;3:824825.
Kosten IJ, Buskermolen JK, Spiekstra SW, de Gruijl TD, Gibbs S. Gingiva equivalents secrete negligible amounts of key chemokines involved in langerhans cell migration compared to skin equivalents. J Immunol Res. 2015;2015:627125.
Fletcher AL, Malhotra D, Acton SE, Lukacs-Kornek V, Bellemare-Pelletier A, Curry M, et al. Reproducible isolation of lymph node stromal cells reveals site-dependent differences in fibroblastic reticular cells. Front Immunol. 2011;2:35.
Masterson AJ, Sombroek CC, De Gruijl TD, Graus YM, van der Vliet HJ, Lougheed SM, et al. Mutz-3, a human cell line model for the cytokine-induced differentiation of dendritic cells from cd34+ precursors. Blood. 2002;100:701–3.
Michielon E, Lopez Gonzalez M, Burm JLA, Waaijman T, Jordanova ES, de Gruijl TD, Gibbs S. Micro-environmental cross-talk in an organotypic human melanoma-in-skin model directs m2-like monocyte differentiation via il-10. Cancer Immunol Immunother. 2020;69:2319–31.
Roet JEG, Mikula AM, Kok Md, Chadick CH, Vallejo JJG, Roest HP, et al. Unbiased method for spectral analysis of cells with great diversity of autofluorescence spectra. BIORXIV. 2023;07:550943.
Renier N, Wu Z, Simon DJ, Yang J, Ariel P, Tessier-Lavigne M. Idisco: a simple, rapid method to immunolabel large tissue samples for volume imaging. Cell. 2014;159:896–910.
Larsson K, Lindstedt M, Borrebaeck CA. Functional and transcriptional profiling of mutz-3, a myeloid cell line acting as a model for dendritic cells. Immunology. 2006;117:156–66.
Eom J, Park SM, Feisst V, Chen CJ, Mathy JE, McIntosh JD, et al. Distinctive subpopulations of stromal cells are present in human lymph nodes infiltrated with melanoma. Cancer Immunol Res. 2020;8:990–1003.
Freynet O, Marchal-Somme J, Jean-Louis F, Mailleux A, Crestani B, Soler P, Michel L. Human lung fibroblasts may modulate dendritic cell phenotype and function: results from a pilot in vitro study. Respir Res. 2016;17:36.
Seguier S, Tartour E, Guerin C, Couty L, Lemitre M, Lallement L, et al. Inhibition of the differentiation of monocyte-derived dendritic cells by human gingival fibroblasts. PLoS ONE. 2013;8:e70937.
Saalbach A, Klein C, Sleeman J, Sack U, Kauer F, Gebhardt C, et al. Dermal fibroblasts induce maturation of dendritic cells. J Immunol. 2007;178:4966–74.
Engering A, van Vliet SJ, Hebeda K, Jackson DG, Prevo R, Singh SK, et al. Dynamic populations of dendritic cell-specific icam-3 grabbing nonintegrin-positive immature dendritic cells and liver/lymph node-specific icam-3 grabbing nonintegrin-positive endothelial cells in the outer zones of the paracortex of human lymph nodes. Am J Pathol. 2004;164:1587–95.
Askmyr D, Abolhalaj M, Gomez Jimenez D, Greiff L, Lindstedt M, Lundberg K. Pattern recognition receptor expression and maturation profile of dendritic cell subtypes in human tonsils and lymph nodes. Hum Immunol. 2021;82:976–81.
Min J, Yang D, Kim M, Haam K, Yoo A, Choi JH, et al. Inflammation induces two types of inflammatory dendritic cells in inflamed lymph nodes. Exp Mol Med. 2018;50:e458.
Park SJ, Nakagawa T, Kitamura H, Atsumi T, Kamon H, Sawa S, et al. Il-6 regulates in vivo dendritic cell differentiation through stat3 activation. J Immunol. 2004;173:3844–54.
Kirkling ME, Cytlak U, Lau CM, Lewis KL, Resteu A, Khodadadi-Jamayran A, et al. Notch signaling facilitates in vitro generation of cross-presenting classical dendritic cells. Cell Rep. 2018;23:3658e6-72e6.
Cheng P, Nefedova Y, Corzo CA, Gabrilovich DI. Regulation of dendritic-cell differentiation by bone marrow stroma via different notch ligands. Blood. 2007;109:507–15.
Fasnacht N, Huang HY, Koch U, Favre S, Auderset F, Chai Q, et al. Specific fibroblastic niches in secondary lymphoid organs orchestrate distinct notch-regulated immune responses. J Exp Med. 2014;211:2265–79.
van Pul KM, Vuylsteke R, van de Ven R, Te Velde EA, Rutgers EJT, van den Tol PM, et al. Selectively hampered activation of lymph node-resident dendritic cells precedes profound t cell suppression and metastatic spread in the breast cancer sentinel lymph node. J Immunother Cancer. 2019;7:133.
van Pul KM, Fransen MF, van de Ven R, de Gruijl TD. Immunotherapy goes local: the central role of lymph nodes in driving tumor infiltration and efficacy. Front Immunol. 2021;12:643291.
Angel CE, Chen CJ, Horlacher OC, Winkler S, John T, Browning J, et al. Distinctive localization of antigen-presenting cells in human lymph nodes. Blood. 2009;113:1257–67.
Jongbloed SL, Kassianos AJ, McDonald KJ, Clark GJ, Ju X, Angel CE, et al. Human cd141+ (bdca-3)+ dendritic cells (dcs) represent a unique myeloid dc subset that cross-presents necrotic cell antigens. J Exp Med. 2010;207:1247–60.
Saalbach A, Klein C, Schirmer C, Briest W, Anderegg U, Simon JC. Dermal fibroblasts promote the migration of dendritic cells. J Invest Dermatol. 2010;130:444–54.
Hoves S, Krause SW, Schutz C, Halbritter D, Scholmerich J, Herfarth H, Fleck M. Monocyte-derived human macrophages mediate anergy in allogeneic t cells and induce regulatory t cells. J Immunol. 2006;177:2691–8.
Ugur M, Labios RJ, Fenton C, Knopper K, Jobin K, Imdahl F, et al. Lymph node medulla regulates the spatiotemporal unfolding of resident dendritic cell networks. Immunity. 2023;56:1778e10-93e10.
Hernandez-Lopez C, Valencia J, Hidalgo L, Martinez VG, Zapata AG, Sacedon R, et al. Cxcl12/cxcr4 signaling promotes human thymic dendritic cell survival regulating the bcl-2/bax ratio. Immunol Lett. 2008;120:72–8.
Tiberio L, Del Prete A, Schioppa T, Sozio F, Bosisio D, Sozzani S. Chemokine and chemotactic signals in dendritic cell migration. Cell Mol Immunol. 2018;15:346–52.
Sixt M, Kanazawa N, Selg M, Samson T, Roos G, Reinhardt DP, et al. The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the t cell area of the lymph node. Immunity. 2005;22:19–29.
van Tienderen GS, van Beek MEA, Schurink IJ, Rosmark O, Roest HP, Tieleman J, et al. Modelling metastatic colonization of cholangiocarcinoma organoids in decellularized lung and lymph nodes. Front Oncol. 2022;12:1101901.
Morgado FN, da Silva AVA, Porrozzi R. Infectious diseases and the lymphoid extracellular matrix remodeling: a focus on conduit system. Cells. 2020;9:725.
Sutherland TE, Dyer DP, Allen JE. The extracellular matrix and the immune system: a mutually dependent relationship. Science. 2023;379:eabp8964.
Choi YS, Jeong E, Lee JS, Kim SK, Jo SH, Kim YG, et al. Immunomodulatory scaffolds derived from lymph node extracellular matrices. ACS Appl Mater Interfaces. 2021;13:14037–49.
Rasaiyaah J, Noursadeghi M, Kellam P, Chain B. Transcriptional and functional defects of dendritic cells derived from the mutz-3 leukaemia line. Immunology. 2009;127:429–41.
Lundberg K, Albrekt AS, Nelissen I, Santegoets S, de Gruijl TD, Gibbs S, Lindstedt M. Transcriptional profiling of human dendritic cell populations and models–unique profiles of in vitro dendritic cells and implications on functionality and applicability. PLoS One. 2013;8:e52875.
Santegoets SJ, van den Eertwegh AJ, van de Loosdrecht AA, Scheper RJ, de Gruijl TD. Human dendritic cell line models for dc differentiation and clinical dc vaccination studies. J Leukoc Biol. 2008;84:1364–73.
Santegoets SJ, Schreurs MW, Masterson AJ, Liu YP, Goletz S, Baumeister H, et al. In vitro priming of tumor-specific cytotoxic t lymphocytes using allogeneic dendritic cells derived from the human mutz-3 cell line. Cancer Immunol Immunother. 2006;55:1480–90.
Santegoets SJ, Masterson AJ, van der Sluis PC, Lougheed SM, Fluitsma DM, van den Eertwegh AJ, et al. A cd34(+) human cell line model of myeloid dendritic cell differentiation: evidence for a cd14(+)cd11b(+) langerhans cell precursor. J Leukoc Biol. 2006;80:1337–44.
Santegoets SJ, Bontkes HJ, Stam AG, Bhoelan F, Ruizendaal JJ, van den Eertwegh AJ, et al. Inducing antitumort cell immunity: comparative functional analysis of interstitial versus langerhans dendritic cells in a human cell line model. J Immunol. 2008;180:4540–9.
Ouwehand K, Spiekstra SW, Waaijman T, Breetveld M, Scheper RJ, de Gruijl TD, Gibbs S. Ccl5 and ccl20 mediate immigration of langerhans cells into the epidermis of full thickness human skin equivalents. Eur J Cell Biol. 2012;91:765–73.
Ouwehand K, Oosterhoff D, Breetveld M, Scheper RJ, de Gruijl TD, Gibbs S. Irritant-induced migration of langerhans cells coincides with an il-10-dependent switch to a macrophage-like phenotype. J Invest Dermatol. 2011;131:418–25.
Park SM, Angel CE, McIntosh JD, Brooks AE, Middleditch M, Chen CJ, et al. Sphingosine-1-phosphate lyase is expressed by cd68+ cells on the parenchymal side of marginal reticular cells in human lymph nodes. Eur J Immunol. 2014;44:2425–36.
Acknowledgements
The presented work was funded by the European Union's Horizon 2020 research and innovation programme under grant agreement No. 847551 ARCAID. AM is supported by NWO ZonMw TOP Grant (91217014). AJA is supported by NWO Veni ZonMw (09150162010163). JJK is supported by NWO LymphChip (1292.19.019). CMdW is supported by Cancer Center Amsterdam (Grant No. CCA2019-9-57 and CCA2020-9-73). LJWvdL is supported by funding from the Convergence Health Technology Flagship grant (Organ Transplantation) and Medical Delta program grant (Regenerative Medicine 4D). The authors would like to thank and acknowledge the expert help of the Microscopy & Cytometry Core Facility at Amsterdam UMC (location Vrije Universiteit Amsterdam). We thank Kim Ober and Dr. Monique Verstegen from the Erasmus MC, Rotterdam for sample logistics.
Author information
Authors and Affiliations
Contributions
Conceptualization: AIM, JJK, CMdW, SG and REM; Methodology: AIM, SWS, JJK, CMdW, SG and REM; Investigation: AIM; Validation: AIM, SWS, AJA, JJK and CMdW; Software: AM and MdK; Data curation: AIM; Writing-original draft preparation: AIM; Writing-review and editing: AIM, JJK, CMdW, SG and REM; Supervision: JJK, CMdW, SG and REM; Funding acquisition: SG and REM; Ethical approval and donor information: LJWvdL and HPR. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflicts of interests.
Ethical statement
HuLNs were obtained from donors during liver transplant procedures performed at the Erasmus MC, Rotterdam, The Netherlands, in accordance to the Medical Ethical Committee (Medisch Ethische Toetsings Commissie; METC) of Erasmus MC (MEC-2014-060). All patients (liver transplant recipients) gave written informed consent to use their donor tissue.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Morrison, A.I., Mikula, A.M., Spiekstra, S.W. et al. An Organotypic Human Lymph Node Model Reveals the Importance of Fibroblastic Reticular Cells for Dendritic Cell Function. Tissue Eng Regen Med 21, 455–471 (2024). https://doi.org/10.1007/s13770-023-00609-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-023-00609-x