Abstract
Background:
Cutaneous wound healing represents a common fundamental phenomenon requiring the participation of cells of distinct types and a major concern for the public. Evidence has confirmed that photobiomodulation (PBM) using near-infrared (NIR) can promote wound healing, but the cells involved and the precise molecular mechanisms remain elusive.
Methods:
Full-thickness skin defects with a diameter of 1.0 cm were made on the back of rats and randomly divided into the control group, 10 J, 15 J, and 30 J groups. The wound healing rate at days 4, 8, and 12 postoperatively was measured. HE and Masson staining was conducted to reveal the histological characteristics. Immunofluorescence staining was performed to label the epidermal stem cells (ESCs) and hair follicle stem cells (HFSCs). Western blot was performed to detect the expressions of proteins associated with ESCs and HFSCs. Cutaneous wound tissues were collected for RNA sequencing. Gene ontology and the Kyoto Encyclopedia of Genes and Genomes analysis was performed, and the hub genes were identified using CytoHubba and validated by qRT-PCR.
Results:
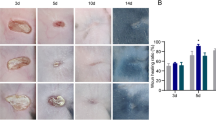
PBM can promote reepithelialization, extracellular matrix deposition, and wound healing, increase the number of KRT14+/PCNA+ ESCs and KRT15+/PCNA+ HFSCs, and upregulate the protein expression of P63, Krt14, and PCNA. Three hundred and sixty-six differentially expressed genes (DEGs) and 7 hub genes including Sox9, Krt5, Epcam, Cdh1, Cdh3, Dsp, and Pkp3 were identified. These DEGs are enriched in skin development, cell junction, and cadherin binding involved in cell–cell adhesion etc., while these hub genes are related to skin derived stem cells and cell adhesion.
Conclusion:
PBM accelerates wound healing by enhancing reepithelialization through promoting ESCs and HFSCs proliferation and elevating the expression of genes associated with stem cells and cell adhesion. This may provide a valuable alternative strategy to promote wound healing and reepithelialization by modulating the proliferation of skin derived stem cells and regulating genes related to cell adhesion.









Similar content being viewed by others
Data availability
The data presented in this study are available on request from all the authors.
References
Guan Y, Yang YJ, Nagarajan P, Ge Y. Transcriptional and signalling regulation of skin epithelial stem cells in homeostasis, wounds and cancer. Exp Dermatol. 2021;30:529–45.
Zhao H, Li Z, Wang Y, Zhou K, Li H, Bi S, et al. Bioengineered MSC-derived exosomes in skin wound repair and regeneration. Front Cell Dev Biol. 2023;11:1029671.
Kimura S, Tsuji T. Mechanical and immunological regulation in wound healing and skin reconstruction. Int J Mol Sci. 2021;22:5474.
Ivanov E, Akhmetshina M, Erdiakov A, Gavrilova S. Sympathetic system in wound healing: multistage control in normal and diabetic skin. Int J Mol Sci. 2023;24:2045.
Nanba D, Toki F, Asakawa K, Matsumura H, Shiraishi K, Sayama K, et al. EGFR-mediated epidermal stem cell motility drives skin regeneration through COL17A1 proteolysis. J Cell Biol. 2021;220:e202012073.
Mao MQ, Jing J, Miao YJ, Lv ZF. Epithelial-mesenchymal interaction in hair regeneration and skin wound healing. Front Med (Lausanne). 2022;9:863786.
Garcin CL, Ansell DM. The battle of the bulge: re-evaluating hair follicle stem cells in wound repair. Exp Dermatol. 2017;26:101–4.
Oyebode O, Houreld NN, Abrahamse H. Photobiomodulation in diabetic wound healing: a review of red and near-infrared wavelength applications. Cell Biochem Funct. 2021;39:596–612.
Mester E, Szende B, Gartner P. The effect of laser beams on the growth of hair in mice. Radiobiol Radiother (Berl). 1968;9:621–6.
Yadav A, Gupta A. Noninvasive red and near-infrared wavelength-induced photobiomodulation: promoting impaired cutaneous wound healing. Photodermatol Photoimmunol Photomed. 2017;33:4–13.
Gungormus M, Akyol UK. Effect of biostimulation on wound healing in diabetic rats. Photomed Laser Surg. 2009;27:607–10.
Akyol U, Gungormus M. The effect of low-level laser therapy on healing of skin incisions made using a diode laser in diabetic rats. Photomed Laser Surg. 2010;28:51–5.
Fekrazad R, Sarrafzadeh A, Kalhori K, Khan I, Arany PR, Giubellino A. Improved wound remodeling correlates with modulated TGF-beta expression in skin diabetic wounds following combined red and infrared photobiomodulation treatments. Photochem Photobiol. 2018;94:775–9.
Demirturk-Gocgun O, Baser U, Aykol-Sahin G, Dinccag N, Issever H, Yalcin F. Role of low-level laser therapy as an adjunct to initial periodontal treatment in type 2 diabetic patients: a split-mouth, randomized controlled clinical trial. Photomed Laser Surg. 2017;35:111–5.
Pereira DA, Mendes P, de Souza SS, de Rezende BG, Pessoa R, de Oliveira G. Effect of the association of infra-red and red wavelength photobiomodulation therapy on the healing of post-extraction sockets of third lower molars: a split-mouth randomized clinical trial. Lasers Med Sci. 2022;37:2479–87.
Amaroli A, Ravera S, Baldini F, Benedicenti S, Panfoli I, Vergani L. Photobiomodulation with 808-nm diode laser light promotes wound healing of human endothelial cells through increased reactive oxygen species production stimulating mitochondrial oxidative phosphorylation. Lasers Med Sci. 2019;34:495–504.
de Abreu P, de Arruda J, Mesquita RA, Abreu LG, Diniz I, Silva TA. Photobiomodulation effects on keratinocytes cultured in vitro: a critical review. Lasers Med Sci. 2019;34:1725–34.
Chen Y, Liu L, Fan J, Zhang T, Zeng Y, Su Z. Low-level laser treatment promotes skin wound healing by activating hair follicle stem cells in female mice. Lasers Med Sci. 2022;37:1699–707.
Al-Watban FA. Laser therapy converts diabetic wound healing to normal healing. Photomed Laser Surg. 2009;27:127–35.
Khan I, Rahman SU, Tang E, Engel K, Hall B, Kulkarni AB, et al. Accelerated burn wound healing with photobiomodulation therapy involves activation of endogenous latent TGF-beta1. Sci Rep. 2021;11:13371.
Prado TP, Zanchetta FC, Barbieri B, Aparecido C, Melo LM, Araujo EP. Photobiomodulation with blue light on wound healing: a scoping review. Life (Basel). 2023;13:575.
Feng J, Li X, Zhu S, Xie Y, Du J, Ge H, et al. Photobiomodulation with 808-nm diode laser enhances gingival wound healing by promoting migration of human gingival mesenchymal stem cells via ROS/JNK/NF-kappaB/MMP-1 pathway. Lasers Med Sci. 2020;35:1831–9.
Gupta A, Dai T, Hamblin MR. Effect of red and near-infrared wavelengths on low-level laser (light) therapy-induced healing of partial-thickness dermal abrasion in mice. Lasers Med Sci. 2014;29:257–65.
Rezende SB, Ribeiro MS, Nunez SC, Garcia VG, Maldonado EP. Effects of a single near-infrared laser treatment on cutaneous wound healing: biometrical and histological study in rats. J Photochem Photobiol B. 2007;87:145–53.
Lau P, Bidin N, Krishnan G, AnaybBaleg SM, Sum MB, Bakhtiar H, et al. Photobiostimulation effect on diabetic wound at different power density of near infrared laser. J Photochem Photobiol B. 2015;151:201–7.
Hu B, Zhao X, Lu Y, Zhu Y, He H. A transient photoactivation of epidermal stem cells by femtosecond laser promotes skin wound healing. J Biophotonics. 2022;15:e202200217.
Prabhu V, Rao BSS, Rao ACK, Prasad K, Mahato KK. Photobiomodulation invigorating collagen deposition, proliferating cell nuclear antigen and Ki67 expression during dermal wound repair in mice. Lasers Med Sci. 2022;37:171–80.
Zhang Y, Su J, Ma K, Li H, Fu X, Zhang C. Photobiomodulation promotes hair regeneration in injured skin by enhancing migration and exosome secretion of dermal papilla cells. Wound Repair Regen. 2022;30:245–57.
Jo A, Denduluri S, Zhang B, Wang Z, Yin L, Yan Z, et al. The versatile functions of Sox9 in development, stem cells, and human diseases. Genes Dis. 2014;1:149–61.
Luanpitpong S, Li J, Manke A, Brundage K, Ellis E, McLaughlin SL, et al. SLUG is required for SOX9 stabilization and functions to promote cancer stem cells and metastasis in human lung carcinoma. Oncogene. 2016;35:2824–33.
Voronkova MA, Luanpitpong S, Rojanasakul LW, Castranova V, Dinu CZ, Riedel H, et al. SOX9 regulates cancer stem-like properties and metastatic potential of single-walled carbon nanotube-exposed cells. Sci Rep. 2017;7:11653.
Qian L, Pi L, Fang BR, Meng XX. Adipose mesenchymal stem cell-derived exosomes accelerate skin wound healing via the lncRNA H19/miR-19b/SOX9 axis. Lab Invest. 2021;101:1254–66.
Bie Q, Zhai R, Chen Y, Li Y, Xie N, Wang B, et al. Sox9 Is crucial for mesenchymal stem cells to enhance cutaneous wound healing. Int J Stem Cells. 2021;14:465–74.
Zhao P, Dang Z, Liu M, Guo D, Luo R, Zhang M, et al. Molecular hydrogen promotes wound healing by inducing early epidermal stem cell proliferation and extracellular matrix deposition. Inflamm Regen. 2023;43:22.
Dekoninck S, Blanpain C. Stem cell dynamics, migration and plasticity during wound healing. Nat Cell Biol. 2019;21:18–24.
Heidari F, Yari A, Teimourian S, Joulai VS, Nobakht M. Effects of hair follicle stem cells coupled with polycaprolactone scaffold on cutaneous wound healing in diabetic male rats. J Surg Res. 2023;281:200–13.
Candi E, Amelio I, Agostini M, Melino G. MicroRNAs and p63 in epithelial stemness. Cell Death Differ. 2015;22:12–21.
McGinn O, Ward AV, Fettig LM, Riley D, Ivie J, Paul KV, et al. Cytokeratin 5 alters beta-catenin dynamics in breast cancer cells. Oncogene. 2020;39:2478–92.
Srivastava SS, Alam H, Patil SJ, Shrinivasan R, Raikundalia S, Chaudhari PR, et al. Keratin 5/14-mediated cell differentiation and transformation are regulated by TAp63 and Notch-1 in oral squamous cell carcinoma-derived cells. Oncol Rep. 2018;39:2393–401.
Liu F, Zhou H, Du W, Huang X, Zheng X, Zhang C, et al. Hair follicle stem cells combined with human allogeneic acellular amniotic membrane for repair of full thickness skin defects in nude mice. J Tissue Eng Regen Med. 2020;14:723–35.
Shimomura Y, Wajid M, Shapiro L, Christiano AM. P-cadherin is a p63 target gene with a crucial role in the developing human limb bud and hair follicle. Development. 2008;135:743–53.
Vasioukhin V, Bowers E, Bauer C, Degenstein L, Fuchs E. Desmoplakin is essential in epidermal sheet formation. Nat Cell Biol. 2001;3:1076–85.
Tewari R, Bailes E, Bunting KA, Coates JC. Armadillo-repeat protein functions: questions for little creatures. Trends Cell Biol. 2010;20:470–81.
Muller L, Rietscher K, Keil R, Neuholz M, Hatzfeld M. Plakophilin 3 phosphorylation by ribosomal S6 kinases supports desmosome assembly. J Cell Sci. 2020;133:238295.
Sklyarova T, Bonne S, D’Hooge P, Denecker G, Goossens S, De Rycke R, et al. Plakophilin-3-deficient mice develop hair coat abnormalities and are prone to cutaneous inflammation. J Invest Dermatol. 2008;128:1375–85.
Muhmer M, Ditthardt D, Jakel J, Wischmann V, Moll R, Schmidt A. An alternative promoter of the human plakophilin-3 gene controls the expression of the new isoform PKP3b. Cell Tissue Res. 2014;355:143–62.
Cadwell CM, Su W, Kowalczyk AP. Cadherin tales: Regulation of cadherin function by endocytic membrane trafficking. Traffic. 2016;17:1262–71.
Abreu-Blanco MT, Watts JJ, Verboon JM, Parkhurst SM. Cytoskeleton responses in wound repair. Cell Mol Life Sci. 2012;69:2469–83.
Plutoni C, Bazellieres E, Le Borgne-Rochet M, Comunale F, Brugues A, Seveno M, et al. P-cadherin promotes collective cell migration via a Cdc42-mediated increase in mechanical forces. J Cell Biol. 2016;212:199–217.
Koizumi M, Matsuzaki T, Ihara S. Expression of P-cadherin distinct from that of E-cadherin in re-epithelialization in neonatal rat skin. Dev Growth Differ. 2005;47:75–85.
Slanchev K, Carney TJ, Stemmler MP, Koschorz B, Amsterdam A, Schwarz H, et al. The epithelial cell adhesion molecule EpCAM is required for epithelial morphogenesis and integrity during zebrafish epiboly and skin development. PLoS Genet. 2009;5:e1000563.
Acknowledgement
This work was supported by Key Research and Development Program of Shaanxi, China (No. 2020SF-179).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical statement
The experimental protocol was approved by the animal experiment ethics committee of the Fourth Military Medical University (Xi’an, China) (Permit no. IACUC-20220302).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, T., Song, Y., Yang, L. et al. Photobiomodulation Facilitates Rat Cutaneous Wound Healing by Promoting Epidermal Stem Cells and Hair Follicle Stem Cells Proliferation. Tissue Eng Regen Med 21, 65–79 (2024). https://doi.org/10.1007/s13770-023-00601-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-023-00601-5