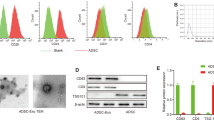
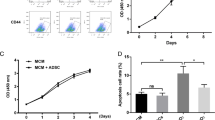
Abstract
Background:
Myocardial infarction (MI) leads to cardiomyocyte death, poor cardiac remodeling, and heart failure, making it a major cause of mortality and morbidity. To restore cardiac pumping function, induction of cardiomyocyte regeneration has become a focus of academic interest. The Hippo pathway is known to regulate cardiomyocyte proliferation and heart size, and its inactivation allows adult cardiomyocytes to re-enter the cell cycle.
Methods:
In this study, we investigated whether exosomes from adipose-derived stem cells (ADSCs) could effectively transfer siRNA for the Hippo pathway regulator Salvador (SAV) into cardiomyocytes to induce cardiomyocyte regeneration in a mouse model of MI.
Results:
Our results showed that exosomes loaded with SAV-siRNA effectively transferred siRNA into cardiomyocytes and induced cardiomyocyte re-entry into the cell cycle, while retaining the previously demonstrated therapeutic efficacy of ADSC-derived exosomes to improve post-infarction cardiac function through anti-fibrotic, pro-angiogenic, and other effects.
Conclusions:
Our findings suggest that siRNA delivery via ADSC-derived exosomes may be a promising approach for the treatment of MI.






Similar content being viewed by others
Data availability statement
The datasets generated and analyzed during the present study are available from the corresponding author on reasonable request.
References
Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the gbd 2019 study. J Am Coll Cardiol. 2020;76:2982–3021.
Sahoo S, Losordo DW. Exosomes and cardiac repair after myocardial infarction. Circ Res. 2014;114:333–44.
Wang J, Liu S, Heallen T, Martin JF. The Hippo pathway in the heart: pivotal roles in development, disease, and regeneration. Nat Rev Cardiol. 2018;15:672–84.
Del Re DP. The hippo signaling pathway: implications for heart regeneration and disease. Clin Transl Med. 2014;3:27.
Zeng Q, Hong W. The emerging role of the hippo pathway in cell contact inhibition, organ size control, and cancer development in mammals. Cancer Cell. 2008;13:188–92.
Xie J, Wang Y, Ai D, Yao L, Jiang H. The role of the Hippo pathway in heart disease. FEBS J. 2022;289:5819–33.
Harvey KF, Hariharan IK. The hippo pathway. Cold Spring Harb Perspect Biol. 2012;4: a011288.
Patel SH, Camargo FD, Yimlamai D. Hippo signaling in the liver regulates organ size, cell fate, and carcinogenesis. Gastroenterology. 2017;152:533–45.
Pan D. The hippo signaling pathway in development and cancer. Dev Cell. 2010;19:491–505.
Calses PC, Crawford JJ, Lill JR, Dey A. Hippo pathway in cancer: aberrant regulation and therapeutic opportunities. Trends Cancer. 2019;5:297–307.
Heallen T, Morikawa Y, Leach J, Tao G, Willerson JT, Johnson RL, et al. Hippo signaling impedes adult heart regeneration. Development. 2013;140:4683–90.
Liu S, Li K, Wagner Florencio L, Tang L, Heallen TR, Leach JP, et al. Gene therapy knockdown of Hippo signaling induces cardiomyocyte renewal in pigs after myocardial infarction. Sci Transl Med 2021;13:eabd6892.
Setten RL, Rossi JJ, Han SP. The current state and future directions of RNAi-based therapeutics. Nat Rev Drug Discov. 2019;18:421–46.
Kim B, Park JH, Sailor MJ. Rekindling RNAi therapy: materials design requirements for in vivo siRNA delivery. Adv Mater. 2019;31: e1903637.
Revia RA, Stephen ZR, Zhang M. Theranostic nanoparticles for RNA-based cancer treatment. Acc Chem Res. 2019;52:1496–506.
Chen X, Mangala LS, Rodriguez-Aguayo C, Kong X, Lopez-Berestein G, Sood AK. RNA interference-based therapy and its delivery systems. Cancer Metastasis Rev. 2018;37:107–24.
Ding Y, Zhang Y, Liu X. Combinational treatments of RNA interference and extracellular vesicles in the spinocerebellar ataxia. Front Mol Neurosci. 2022;15:1043947.
Ju Z, Ma J, Wang C, Yu J, Qiao Y, Hei F. Exosomes from iPSCs delivering siRNA attenuate intracellular adhesion molecule-1 expression and neutrophils adhesion in pulmonary microvascular endothelial cells. Inflammation. 2017;40:486–96.
Shtam TA, Kovalev RA, Varfolomeeva EY, Makarov EM, Kil YV, Filatov MV. Exosomes are natural carriers of exogenous siRNA to human cells in vitro. Cell Commun Signal. 2013;11:88.
Hinderer C, Katz N, Buza EL, Dyer C, Goode T, Bell P, et al. Severe toxicity in nonhuman primates and piglets following high-dose intravenous administration of an adeno-associated virus vector expressing human SMN. Hum Gene Ther. 2018;29:285–98.
Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science 2020;367.
Huang W, Qu M, Li L, Liu T, Lin M, Yu X. SiRNA in MSC-derived exosomes silences CTGF gene for locomotor recovery in spinal cord injury rats. Stem Cell Res Ther. 2021;12:334.
Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29:341–5.
Kimiz-Gebologlu I, Oncel SS. Exosomes: large-scale production, isolation, drug loading efficiency, and biodistribution and uptake. J Control Release. 2022;347:533–43.
Liang Y, Duan L, Lu J, Xia J. Engineering exosomes for targeted drug delivery. Theranostics. 2021;11:3183–95.
Batrakova EV, Kim MS. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J Control Release. 2015;219:396–405.
Tian M, Ticer T, Wang Q, Walker S, Pham A, Suh A, et al. Adipose-derived biogenic nanoparticles for suppression of inflammation. Small. 2020;16: e1904064.
Chang TH, Wu CS, Chiou SH, Chang CH, Liao HJ. Adipose-derived stem cell exosomes as a novel anti-inflammatory agent and the current therapeutic targets for rheumatoid arthritis. Biomedicines 2022;10:1725.
Liu Z, Wang S, Tapeinos C, Torrieri G, Känkänen V, El-Sayed N, et al. Non-viral nanoparticles for RNA interference: Principles of design and practical guidelines. Adv Drug Deliv Rev. 2021;174:576–612.
Deng S, Zhou X, Ge Z, Song Y, Wang H, Liu X, et al. Exosomes from adipose-derived mesenchymal stem cells ameliorate cardiac damage after myocardial infarction by activating S1P/SK1/S1PR1 signaling and promoting macrophage M2 polarization. Int J Biochem Cell Biol. 2019;114: 105564.
Liu Z, Xu Y, Wan Y, Gao J, Chu Y, Li J. Exosomes from adipose-derived mesenchymal stem cells prevent cardiomyocyte apoptosis induced by oxidative stress. Cell Death Discov. 2019;5:79.
Mao C, Li D, Zhou E, Gao E, Zhang T, Sun S, et al. Extracellular vesicles from anoxia preconditioned mesenchymal stem cells alleviate myocardial ischemia/reperfusion injury. Aging (Albany NY). 2021;13:6156–70.
An YH, Kim DH, Lee EJ, Lee D, Park MJ, Ko J, et al. High-efficient production of adipose-derived stem cell (ADSC) secretome through maturation process and its non-scarring wound healing applications. Front Bioeng Biotechnol. 2021;9: 681501.
Bai X, Yan Y, Song YH, Seidensticker M, Rabinovich B, Metzele R, et al. Both cultured and freshly isolated adipose tissue-derived stem cells enhance cardiac function after acute myocardial infarction. Eur Heart J. 2010;31:489–501.
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells the international society for cellular therapy position statement. Cytotherapy. 2006;8:315–7.
Han M, Gu Y, Lu P, Li J, Cao H, Li X, et al. Exosome-mediated lncRNA AFAP1-AS1 promotes trastuzumab resistance through binding with AUF1 and activating ERBB2 translation. Mol Cancer. 2020;19:26.
Witwer KW, Goberdhan DC, O’Driscoll L, Thery C, Welsh JA, Blenkiron C, et al. Updating MISEV: evolving the minimal requirements for studies of extracellular vesicles. J Extracell Vesicles. 2021;10:e12182.
Wang J, Wang Y, Duan Z, Hu W. Hypoxia-induced alterations of transcriptome and chromatin accessibility in HL-1 cells. IUBMB Life. 2020;72:1737–46.
Chen J, Huang ZP, Seok HY, Ding J, Kataoka M, Zhang Z, et al. mir-17-92 cluster is required for and sufficient to induce cardiomyocyte proliferation in postnatal and adult hearts. Circ Res. 2013;112:1557–66.
Zhao J, Li X, Hu J, Chen F, Qiao S, Sun X, et al. Mesenchymal stromal cell-derived exosomes attenuate myocardial ischaemia-reperfusion injury through miR-182-regulated macrophage polarization. Cardiovasc Res. 2019;115:1205–16.
Rostamzadeh F, Shadkam-Farrokhi M, Jafarinejad-Farsangi S, Najafipour H, Ansari-Asl Z, Yeganeh-Hajahmadi M. PEGylated graphene quantum dot improved cardiac function in rats with myocardial infarction: morphological, oxidative stress, and toxicological evidences. Oxid Med Cell Longev. 2021;2021:8569225.
Germanova E, Khmil N, Pavlik L, Mikheeva I, Mironova G, Lukyanova L. The role of mitochondrial enzymes, succinate-coupled signaling pathways and mitochondrial ultrastructure in the formation of urgent adaptation to acute hypoxia in the myocardium. Int J Mol Sci. 2022;23:14248.
Feng J, Zhan J, Ma S. LRG1 promotes hypoxia-induced cardiomyocyte apoptosis and autophagy by regulating hypoxia-inducible factor-1alpha. Bioengineered. 2021;12:8897–907.
Liu J, Jiang M, Deng S, Lu J, Huang H, Zhang Y, et al. miR-93-5p-containing exosomes treatment attenuates acute myocardial infarction-induced myocardial damage. Mol Ther Nucleic Acids. 2018;11:103–15.
Li Y, Feng J, Song S, Li H, Yang H, Zhou B, et al. gp130 controls cardiomyocyte proliferation and heart regeneration. Circulation. 2020;142:967–82.
Wang Y, Li Y, Feng J, Liu W, Li Y, Liu J, et al. Mydgf promotes cardiomyocyte proliferation and Neonatal Heart regeneration. Theranostics. 2020;10:9100–12.
Xin M, Kim Y, Sutherland LB, Murakami M, Qi X, McAnally J, et al. Hippo pathway effector Yap promotes cardiac regeneration. Proc Natl Acad Sci U S A. 2013;110:13839–44.
Tian Y, Liu Y, Wang T, Zhou N, Kong J, Chen L, et al. A microRNA-Hippo pathway that promotes cardiomyocyte proliferation and cardiac regeneration in mice. Sci Transl Med. 2015;7:279ra38.
Meng F, Xie B, Martin JF. Targeting the Hippo pathway in heart repair. Cardiovasc Res. 2022;118:2402–14.
Mia MM, Singh MK. The hippo signaling pathway in cardiac development and diseases. Front Cell Dev Biol. 2019;7:211.
Gao C, Wang Y. YAP: The nexus between metabolism and cardiac remodeling. J Clin Invest 2022;132:e157664.
Chong SY, Lee CK, Huang C, Ou YH, Charles CJ, Richards AM, et al. Extracellular vesicles in cardiovascular diseases: alternative biomarker sources, therapeutic agents, and drug delivery xarriers. Int J Mol Sci 2019;20:3272.
Rochette L, Mazini L, Malka G, Zeller M, Cottin Y, Vergely C. The crosstalk of adipose-derived stem cells (ADSC), oxidative stress, and inflammation in protective and adaptive responses. Int J Mol Sci 2020;21:9262.
Castiglione F, Dewulf K, Hakim L, Weyne E, Montorsi F, Russo A, et al. Adipose-derived stem cells counteract urethral stricture formation in rats. Eur Urol. 2016;70:1032–41.
Mazini L, Rochette L, Amine M, Malka G. Regenerative capacity of adipose derived stem cells (ADSCs), comparison with mesenchymal stem cells (MSCs). Int J Mol Sci 2019;20:2523.
Zhang X, Jiang Y, Huang Q, Wu Z, Pu H, Xu Z, et al. Exosomes derived from adipose-derived stem cells overexpressing glyoxalase-1 protect endothelial cells and enhance angiogenesis in type 2 diabetic mice with limb ischemia. Stem Cell Res Ther. 2021;12:403.
Wu J, Yang Q, Wu S, Yuan R, Zhao X, Li Y, et al. Adipose-derived stem cell exosomes promoted hair regeneration. Tissue Eng Regen Med. 2021;18:685–91.
Lin J, Wang Z, Huang J, Tang S, Saiding Q, Zhu Q, et al. Microenvironment-protected exosome-hydrogel for facilitating endometrial regeneration, fertility restoration, and live birth of offspring. Small. 2021;17:e2007235.
Wang T, Li T, Niu X, Hu L, Cheng J, Guo D, et al. ADSC-derived exosomes attenuate myocardial infarction injury by promoting miR-205-mediated cardiac angiogenesis. Biol Direct. 2023;18:6.
Luo Q, Guo D, Liu G, Chen G, Hang M, Jin M. Exosomes from MiR-126-overexpressing Adscs are therapeutic in relieving acute myocardial ischaemic injury. Cell Physiol Biochem. 2017;44:2105–16.
Pan J, Alimujiang M, Chen Q, Shi H, Luo X. Exosomes derived from miR-146a-modified adipose-derived stem cells attenuate acute myocardial infarction-induced myocardial damage via downregulation of early growth response factor 1. J Cell Biochem. 2019;120:4433–43.
Wei Z, Chen Z, Zhao Y, Fan F, Xiong W, Song S, et al. Mononuclear phagocyte system blockade using extracellular vesicles modified with CD47 on membrane surface for myocardial infarction reperfusion injury treatment. Biomaterials. 2021;275:121000.
Wang X, Chen Y, Zhao Z, Meng Q, Yu Y, Sun J, et al. Engineered exosomes with ischemic myocardium-targeting peptide for targeted therapy in myocardial infarction. J Am Heart Assoc. 2018;7:e008737.
Chen P, Wang L, Fan X, Ning X, Yu B, Ou C, et al. Targeted delivery of extracellular vesicles in heart injury. Theranostics. 2021;11:2263–77.
Wang P, Zhou Y, Richards AM. Effective tools for RNA-derived therapeutics: siRNA interference or miRNA mimicry. Theranostics. 2021;11:8771–96.
Acknowledgements
Not applicable.
Author information
Authors and Affiliations
Contributions
WZB contributed the central idea, analysed most of the data, and wrote the initial draft of the paper. TCZ, JBZ, YL, XH and GL contributed to refining the ideas, carrying out additional analyses and finalizing this paper.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Statement
All animal experiments were approved by the Animal Research Committee of the Fifth Affiliated Hospital of Sun Yat-sen University (No. 00243), in compliance with the “Guidelines for the Care and Use of Experimental Animals” of the National Institutes of Health (NIH Publication, 8th Edition, 2011). All donors were adults and signed an informed consent form.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bai, W., Zhu, T., Zuo, J. et al. Delivery of SAV-siRNA via Exosomes from Adipose-Derived Stem Cells for the Treatment of Myocardial Infarction. Tissue Eng Regen Med 20, 1063–1077 (2023). https://doi.org/10.1007/s13770-023-00588-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-023-00588-z