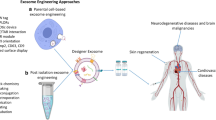
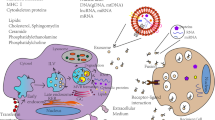
Abstract
Cell-based therapies have been used as promising treatments for several untreatable diseases. However, cell-based therapies have side effects such as tumorigenesis and immune responses. To overcome these side effects, therapeutic effects of exosomes have been researched as replacements for cell-based therapies. In addition, exosomes reduced the risk that can be induced by cell-based therapies. Exosomes contain biomolecules such as proteins, lipids, and nucleic acids that play an essential role in cell–cell and cell–matrix interactions during biological processes. Since the introduction of exosomes, those have been proven perpetually as one of the most effective and therapeutic methods for incurable diseases. Much research has been conducted to enhance the properties of exosomes, including immune regulation, tissue repair, and regeneration. However, yield rate of exosomes is the critical obstacle that should be overcome for practical cell-free therapy. Three-dimensional (3D) culture methods are introduced as a breakthrough to get higher production yields of exosomes. For example, hanging drop and microwell were well known 3D culture methods and easy to use without invasiveness. However, these methods have limitation in mass production of exosomes. Therefore, a scaffold, spinner flask, and fiber bioreactor were introduced for mass production of exosomes isolated from various cell types. Furthermore, exosomes treatments derived from 3D cultured cells showed enhanced cell proliferation, angiogenesis, and immunosuppressive properties. This review provides therapeutic applications of exosomes using 3D culture methods.






Similar content being viewed by others
References
Kusuma GD, Carthew J, Lim R, Frith JE. Effect of the microenvironment on mesenchymal stem cell paracrine signaling: opportunities to engineer the therapeutic effect. Stem Cells Dev. 2017;26:617–31.
Kalra H, Drummen GP, Mathivanan S. Focus on extracellular vesicles: introducing the next small big thing. Int J Mol Sci. 2016;17:170.
Gurung S, Perocheau D, Touramanidou L, Baruteau J. The exosome journey: from biogenesis to uptake and intracellular signalling. Cell Commun Signal. 2021;19:47.
Zhang Y, Liu Y, Liu H, Tang WH. Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci. 2019;9:19.
Huang X, Yuan T, Tschannen M, Sun Z, Jacob H, Du M, et al. Characterization of human plasma-derived exosomal RNAs by deep sequencing. BMC Genomics. 2013;14:1–14.
Hade MD, Suire CN, Suo Z. Mesenchymal stem cell-derived exosomes: applications in regenerative medicine. Cells. 2021;10:1959.
Zhang B, Wang M, Gong A, Zhang X, Wu X, Zhu Y, et al. HucMSC-exosome mediated-Wnt4 signaling is required for cutaneous wound healing. Stem cells. 2015;33:2158–68.
Long Q, Upadhya D, Hattiangady B, Kim DK, An SY, Shuai B, et al. Intranasal MSC-derived A1-exosomes ease inflammation, and prevent abnormal neurogenesis and memory dysfunction after status epilepticus. Proc Natl Acad Sci U S A. 2017;114:E3536–45.
Katsuda T, Tsuchiya R, Kosaka N, Yoshioka Y, Takagaki K, Oki K, et al. Human adipose tissue-derived mesenchymal stem cells secrete functional neprilysin-bound exosomes. Sci Rep. 2013;3:1197.
Dai S, Wei D, Wu Z, Zhou X, Wei X, Huang H, et al. Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer. Mol Ther. 2008;16:782–90.
Haraszti RA, Miller R, Stoppato M, Sere YY, Coles A, Didiot MC, et al. Exosomes produced from 3D cultures of MSCs by tangential flow filtration show higher yield and improved activity. Mol Ther. 2018;26:2838–47.
Yamada KM, Cukierman E. Modeling tissue morphogenesis and cancer in 3D. Cell. 2007;130:601–10.
Shri M, Agrawal H, Rani P, Singh D, Onteru SK. Hanging drop, a best three-dimensional (3D) culture method for primary buffalo and sheep hepatocytes. Sci Rep. 2017;7:1203.
Gidrol X, Fouqué B, Ghenim L, Haguet V, Picollet-D’hahan N, Schaack B. 2D and 3D cell microarrays in pharmacology. Curr Opinion Pharmacol. 2009;9:664–8.
Todorov L, VadeBoncouer T. Etiology and use of the “hanging drop” technique: A review. Pain Res Treat. 2014;2014:146750.
Foglietta F, Canaparo R, Muccioli G, Terreno E, Serpe L. Methodological aspects and pharmacological applications of three-dimensional cancer cell cultures and organoids. Life Sci. 2020;254:117784.
Yu W, Li S, Guan X, Zhang N, Xie X, Zhang K, et al. Higher yield and enhanced therapeutic effects of exosomes derived from MSCs in hydrogel-assisted 3D culture system for bone regeneration. Biomater Adv. 2022;133:112646.
Cao J, Wang B, Tang T, Lv L, Ding Z, Li Z, et al. Three-dimensional culture of MSCs produces exosomes with improved yield and enhanced therapeutic efficacy for cisplatin-induced acute kidney injury. Stem Cell Res Ther. 2020;11:206.
Thippabhotla S, Zhong C, He M. 3D cell culture stimulates the secretion of in vivo like extracellular vesicles. Sci Rep. 2019;9:13012.
Lei Y, Schaffer DV. A fully defined and scalable 3D culture system for human pluripotent stem cell expansion and differentiation. Proc Natl Acad Sci U S A. 2013;110:E5039–48.
Faruqu FN, Liam-Or R, Zhou S, Nip R, Al-Jamal KT. Defined serum-free three-dimensional culture of umbilical cord-derived mesenchymal stem cells yields exosomes that promote fibroblast proliferation and migration in vitro. FASEB J. 2021;35:e21206.
Moshksayan K, Kashaninejad N, Warkiani ME, Lock JG, Moghadas H, Firoozabadi B, et al. Spheroids-on-a-chip: Recent advances and design considerations in microfluidic platforms for spheroid formation and culture. Sens Actuators B Chem. 2018;263:151–76.
Ryu NE, Lee SH, Park H. Spheroid culture system methods and applications for mesenchymal stem cells. Cells. 2019;8:1620.
De Groot T, Veserat K, Berthier E, Beebe D, Theberge A. Surface-tension driven open microfluidic platform for hanging droplet culture. Lab Chip. 2016;16:334–44.
Timmins NE, Nielsen LK. Generation of multicellular tumor spheroids by the hanging-drop method. Methods Mol Med. 2007;140:141–51.
Kim M, Yun HW, Park DY, Choi BH, Min BH. Three-dimensional spheroid culture increases exosome secretion from mesenchymal stem cells. Tissue Eng Regen Med. 2018;15:427–36.
Giusti I, Poppa G, D’Ascenzo S, Esposito L, Vitale AR, Calvisi G, et al. Cancer three-dimensional spheroids mimic in vivo tumor features, displaying “inner” extracellular vesicles and vasculogenic mimicry. Int J Mol Sci. 2022;23:11782.
Manzoor AA, Romita L, Hwang DK. A review on microwell and microfluidic geometric array fabrication techniques and its potential applications in cellular studies. Can J Chem Eng. 2021;99:61–96.
Faruqu FN, Zhou S, Sami N, Gheidari F, Lu H, Al-Jamal KT. Three-dimensional culture of dental pulp pluripotent-like stem cells (DPPSCs) enhances Nanog expression and provides a serum-free condition for exosome isolation. FASEB BioAdv. 2020;2:419–33.
Huang R, Wang J, Chen H, Shi X, Wang X, Zhu Y, et al. The topography of fibrous scaffolds modulates the paracrine function of Ad-MSCs in the regeneration of skin tissues. Biomater Sci. 2019;7:4248–59.
Su N, Gao P-L, Wang K, Wang J-Y, Zhong Y, Luo Y. Fibrous scaffolds potentiate the paracrine function of mesenchymal stem cells: A new dimension in cell-material interaction. Biomaterials. 2017;141:74–85.
Fukuda J, Khademhosseini A, Yeo Y, Yang X, Yeh J, Eng G, et al. Micromolding of photocrosslinkable chitosan hydrogel for spheroid microarray and co-cultures. Biomaterials. 2006;27:5259–67.
Han M, Yang H, Lu X, Li Y, Liu Z, Li F, et al. Three-dimensional-cultured MSC-derived exosome-hydrogel hybrid microneedle array patch for spinal cord repair. Nano Lett. 2022;22:6391–401.
Han M, Zhang Z, Liu Z, Liu Y, Zhao H, Wang B, et al. Three-dimensional-cultured MSC-derived exosome with hydrogel for cerebral ischemia repair. Biomater Adv. 2023;149:213396.
Liu X, Holzwarth JM, Ma PX. Functionalized synthetic biodegradable polymer scaffolds for tissue engineering. Macromol Biosci. 2012;12:911–9.
Xie Y, Kawazoe N, Yang Y, Chen G. Preparation of mesh-like collagen scaffolds for tissue engineering. Mater Adv. 2022;3:1556–64.
Gao W, Liang T, He R, Ren J, Yao H, Wang K, et al. Exosomes from 3D culture of marrow stem cells enhances endothelial cell proliferation, migration, and angiogenesis via activation of the HMGB1/AKT pathway. Stem Cell Res. 2021;50:102122.
Yang L, Zhai Y, Hao Y, Zhu Z, Cheng G. The regulatory functionality of exosomes derived from hUMSCs in 3D culture for Alzheimer’s disease therapy. Small. 2020;16:e1906273.
Gloeckner H, Jonuleit T, Lemke H-D. Monitoring of cell viability and cell growth in a hollow-fiber bioreactor by use of the dye Alamar Blue. J Immunol Methods. 2001;252:131–8.
Yan L, Wu X. Exosomes produced from 3D cultures of umbilical cord mesenchymal stem cells in a hollow-fiber bioreactor show improved osteochondral regeneration activity. Cell Biol Toxicol. 2020;36:165–78.
Clément V, Roy V, Paré B, Goulet CR, Deschênes LT, Berthod F, et al. Tridimensional cell culture of dermal fibroblasts promotes exosome-mediated secretion of extracellular matrix proteins. Sci Rep. 2022;12:19786.
Hu S, Li Z, Cores J, Huang K, Su T, Dinh PU, et al. Needle-free injection of exosomes derived from human dermal fibroblast spheroids ameliorates skin photoaging. ACS Nano. 2019;13:11273–82.
Jovic D, Yu Y, Wang D, Wang K, Li H, Xu F, et al. A brief overview of global trends in MSC-based cell therapy. Stem Cell Rev Rep. 2022;18:1525–45.
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7.
Wang HS, Hung SC, Peng ST, Huang CC, Wei HM, Guo YJ, et al. Mesenchymal stem cells in the Wharton’s jelly of the human umbilical cord. Stem cells. 2004;22:1330–7.
Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–95.
Afizah H, Yang Z, Hui JH, Ouyang H-W, Lee E-H. A comparison between the chondrogenic potential of human bone marrow stem cells (BMSCs) and adipose-derived stem cells (ADSCs) taken from the same donors. Tissue Eng. 2007;13:659–66.
Huang JI, Kazmi N, Durbhakula MM, Hering TM, Yoo JU, Johnstone B. Chondrogenic potential of progenitor cells derived from human bone marrow and adipose tissue: A patient-matched comparison. J Orthop Res. 2005;23:1383–9.
Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–19.
Tomasoni S, Longaretti L, Rota C, Morigi M, Conti S, Gotti E, et al. Transfer of growth factor receptor mRNA via exosomes unravels the regenerative effect of mesenchymal stem cells. Stem Cells Dev. 2013;22:772–80.
Xin H, Li Y, Liu Z, Wang X, Shang X, Cui Y, et al. MiR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem cells. 2013;31:2737–46.
Feng Y, Huang W, Wani M, Yu X, Ashraf M. Ischemic preconditioning potentiates the protective effect of stem cells through secretion of exosomes by targeting Mecp2 via miR-22. PLoS One. 2014;9:e88685.
Del Fattore A, Luciano R, Saracino R, Battafarano G, Rizzo C, Pascucci L, et al. Differential effects of extracellular vesicles secreted by mesenchymal stem cells from different sources on glioblastoma cells. Expert Opin Biol Ther. 2015;15:495–504.
Lopez-Verrilli MA, Caviedes A, Cabrera A, Sandoval S, Wyneken U, Khoury M. Mesenchymal stem cell-derived exosomes from different sources selectively promote neuritic outgrowth. Neuroscience. 2016;320:129–39.
Phinney DG, Pittenger MF. Concise review: MSC-derived exosomes for cell-free therapy. Stem cells. 2017;35:851–8.
Zhang L, Jiao G, Ren S, Zhang X, Li C, Wu W, et al. Exosomes from bone marrow mesenchymal stem cells enhance fracture healing through the promotion of osteogenesis and angiogenesis in a rat model of nonunion. Stem Cell Res Ther. 2020;11:38.
Feng Y, Ke J, Cao P, Deng M, Li J, Cai H, et al. HMGB1-induced angiogenesis in perforated disc cells of human temporomandibular joint. J Cell Mol Med. 2018;22:1283–91.
Dinh PUC, Paudel D, Brochu H, Popowski KD, Gracieux MC, Cores J, et al. Inhalation of lung spheroid cell secretome and exosomes promotes lung repair in pulmonary fibrosis. Nat Commun. 2020;11:1064.
Romanov YA, Volgina N, Vtorushina V, Romanov AY, Dugina T, Kabaeva N, et al. Comparative analysis of secretome of human umbilical cord-and bone marrow-derived multipotent mesenchymal stromal cells. Bull Exp Biol Med. 2019;166:535–40.
Ding M, Shen Y, Wang P, Xie Z, Xu S, Zhu Z, et al. Exosomes isolated from human umbilical cord mesenchymal stem cells alleviate neuroinflammation and reduce amyloid-beta deposition by modulating microglial activation in Alzheimer’s disease. Neurochem Res. 2018;43:2165–77.
Cui GH, Wu J, Mou FF, Xie WH, Wang FB, Wang QL, et al. Exosomes derived from hypoxia-preconditioned mesenchymal stromal cells ameliorate cognitive decline by rescuing synaptic dysfunction and regulating inflammatory responses in APP/PS1 mice. FASEB J. 2018;32:654–68.
Anttonen T, Kirjavainen A, Belevich I, Laos M, Richardson WD, Jokitalo E, et al. Cdc42-dependent structural development of auditory supporting cells is required for wound healing at adulthood. Sci Rep. 2012;2:978.
Kitajima Y, Luo L, Yan C, Tateishi S, Ono Y, Urata Y, et al. Potency of umbilical cord blood-and Wharton’s jelly-derived mesenchymal stem cells for scarless wound healing. Sci Rep. 2016;6:18844.
Zelman-Toister E, Bakos E, Cohen S, Zigmond E, Shezen E, Grabovsky V, et al. CD151 regulates T-cell migration in health and inflammatory bowel disease. Inflamm Bowel Dis. 2016;22:257–67.
Wang XJ, Han G, Owens P, Siddiqui Y, Li AG. Role of TGFβ-mediated inflammation in cutaneous wound healing. J Investig Dermatol Symp Proc. 2006;11:112–7.
Camões SP, Bulut O, Yazar V, Gaspar MM, Simões S, Ferreira R, et al. 3D-MSCs A151 ODN-loaded exosomes are immunomodulatory and reveal a proteomic cargo that sustains wound resolution. J Adv Res. 2022;41:113–28.
Guo SA, Dipietro LA. Factors affecting wound healing. J Dent Res. 2010;89:219–29.
Ren Y, Liu J, Xu H, Wang S, Li S, Xiang M, et al. Knockout of integrin β1 in induced pluripotent stem cells accelerates skin-wound healing by promoting cell migration in extracellular matrix. Stem Cell Res Ther. 2022;13:389.
Miceli V, Pampalone M, Vella S, Carreca AP, Amico G, Conaldi PG. Comparison of immunosuppressive and angiogenic properties of human amnion-derived mesenchymal stem cells between 2D and 3D culture systems. Stem cells Int. 2019;2019:7486279.
Barlow S, Brooke G, Chatterjee K, Price G, Pelekanos R, Rossetti T, et al. Comparison of human placenta-and bone marrow–derived multipotent mesenchymal stem cells. Stem Cells Dev. 2008;17:1095–108.
Bilic G, Zeisberger SM, Mallik AS, Zimmermann R, Zisch AH. Comparative characterization of cultured human term amnion epithelial and mesenchymal stromal cells for application in cell therapy. Cell Transplant. 2008;17:955–68.
Shu J, He X, Li H, Liu X, Qiu X, Zhou T, et al. The beneficial effect of human amnion mesenchymal cells in inhibition of inflammation and induction of neuronal repair in EAE mice. J Immunol Res. 2018;2018:5083797.
Tuca AC, Ertl J, Hingerl K, Pichlsberger M, Fuchs J, Wurzer P, et al. Comparison of matrigel and matriderm as a carrier for human amnion-derived mesenchymal stem cells in wound healing. Placenta. 2016;48:99–103.
Shen WC, Lai YC, Li LH, Liao K, Lai HC, Kao SY, et al. Methylation and PTEN activation in dental pulp mesenchymal stem cells promotes osteogenesis and reduces oncogenesis. Nat Commun. 2019;10:2226.
Alge DL, Zhou D, Adams LL, Wyss BK, Shadday MD, Woods EJ, et al. Donor-matched comparison of dental pulp stem cells and bone marrow-derived mesenchymal stem cells in a rat model. J Tissue Eng Regen Med. 2010;4:73–81.
Shen Z, Kuang S, Zhang Y, Yang M, Qin W, Shi X, et al. Chitosan hydrogel incorporated with dental pulp stem cell-derived exosomes alleviates periodontitis in mice via a macrophage-dependent mechanism. Bioact Mater. 2020;5:1113–26.
Zhuang Z, Yoshizawa-Smith S, Glowacki A, Maltos K, Pacheco C, Shehabeldin M, et al. Induction of M2 macrophages prevents bone loss in murine periodontitis models. J Dent Res. 2019;98:200–8.
Zhang Y, Chen J, Fu H, Kuang S, He F, Zhang M, et al. Exosomes derived from 3D-cultured MSCs improve therapeutic effects in periodontitis and experimental colitis and restore the Th17 cell/Treg balance in inflamed periodontium. Int J Oral Sci. 2021;13:43.
Suh JS, Lee SH, Fouladian Z, Lee JY, Kim T, Kang MK, et al. Rosuvastatin prevents the exacerbation of atherosclerosis in ligature-induced periodontal disease mouse model. Sci Rep. 2020;10:6383.
Tian J, Zhu Q, Zhang Y, Bian Q, Hong Y, Shen Z, et al. Olfactory ecto-mesenchymal stem cell-derived exosomes ameliorate experimental colitis via modulating Th1/Th17 and Treg cell responses. Front Immunol. 2020;11:598322.
Zheng Y, Dong C, Yang J, Jin Y, Zheng W, Zhou Q, et al. Exosomal microRNA-155-5p from PDLSCs regulated Th17/Treg balance by targeting sirtuin-1 in chronic periodontitis. J Cell Physiol. 2019;234:20662–74.
Yang G, Chen Q, Wen D, Chen Z, Wang J, Chen G, et al. A therapeutic microneedle patch made from hair-derived keratin for promoting hair regrowth. ACS Nano. 2019;13:4354–60.
Enshell-Seijffers D, Lindon C, Kashiwagi M, Morgan BA. β-catenin activity in the dermal papilla regulates morphogenesis and regeneration of hair. Dev Cell. 2010;18:633–42.
Rajendran RL, Gangadaran P, Bak SS, Oh JM, Kalimuthu S, Lee HW, et al. Extracellular vesicles derived from MSCs activates dermal papilla cell in vitro and promotes hair follicle conversion from telogen to anagen in mice. Sci Rep. 2017;7:15560.
Higgins CA, Chen JC, Cerise JE, Jahoda CA, Christiano AM. Microenvironmental reprogramming by three-dimensional culture enables dermal papilla cells to induce de novo human hair-follicle growth. Proc Natl Acad Sci U S A. 2013;110:19679–88.
Zhang H, Zhu N-X, Huang K, Cai B-Z, Zeng Y, Xu Y-M, et al. iTRAQ-based quantitative proteomic comparison of early-and late-passage human dermal papilla cell secretome in relation to inducing hair follicle regeneration. PLoS One. 2016;11:e0167474.
Chen Y, Huang J, Chen R, Yang L, Wang J, Liu B, et al. Sustained release of dermal papilla-derived extracellular vesicles from injectable microgel promotes hair growth. Theranostics. 2020;10:1454–1478.
Hu S, Li Z, Lutz H, Huang K, Su T, Cores J, et al. Dermal exosomes containing miR-218–5p promote hair regeneration by regulating β-catenin signaling. Sci Adv. 2020;6:eaba1685.
Ito Y, Hamazaki TS, Ohnuma K, Tamaki K, Asashima M, Okochi H. Isolation of murine hair-inducing cells using the cell surface marker prominin-1/CD133. J Invest Dermatol. 2007;127:1052–60.
Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W. β-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 2001;105:533–45.
Zhou L, Wang H, Jing J, Yu L, Wu X, Lu Z. Morroniside regulates hair growth and cycle transition via activation of the Wnt/β-catenin signaling pathway. Sci Rep. 2018;8:13785.
Rojas M, Xu J, Woods CR, Mora AL, Spears W, Roman J, et al. Bone marrow–derived mesenchymal stem cells in repair of the injured lung. Am J Respir Cell Mol Biol. 2005;33:145–52.
Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, et al. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369–77.
Wagers AJ, Sherwood RI, Christensen JL, Weissman IL. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science. 2002;297:2256–9.
Bruscia EM, Ziegler EC, Price JE, Weiner S, Egan ME, Krause DS. Engraftment of donor-derived epithelial cells in multiple organs following bone marrow transplantation into newborn mice. Stem cells. 2006;24:2299–308.
Henry E, Cores J, Hensley MT, Anthony S, Vandergriff A, de Andrade JB, et al. Adult lung spheroid cells contain progenitor cells and mediate regeneration in rodents with bleomycin-induced pulmonary fibrosis. Stem Cells Transl Med. 2015;4:1265–74.
Cores J, Dinh P-UC, Hensley T, Adler KB, Lobo LJ, Cheng K. A pre-investigational new drug study of lung spheroid cell therapy for treating pulmonary fibrosis. Stem Cells Transl Med. 2020;9:786–98.
Cores J, Hensley MT, Kinlaw K, Rikard SM, Dinh P-U, Paudel D, et al. Safety and efficacy of allogeneic lung spheroid cells in a mismatched rat model of pulmonary fibrosis. Stem Cells Transl Med. 2017;6:1905–16.
Dinh PU-C, Cores J, Hensley MT, Vandergriff AC, Tang J, Allen TA, et al. Derivation of therapeutic lung spheroid cells from minimally invasive transbronchial pulmonary biopsies. Respir Res. 2017;18:132.
Roach KM, Wulff H, Feghali-Bostwick C, Amrani Y, Bradding P. Increased constitutive αSMA and Smad2/3 expression in idiopathic pulmonary fibrosis myofibroblasts is KCa3. 1-dependent. Respir Res. 2014;15:155.
Jia L, Liu Y, Han Y, Zhou X, Wang F. Differential expression and inhibitory effects of aquaporins on the development of adenomyosis. Mol Med Rep. 2020;22:3840–50.
Gauldie J, Bonniaud P, Sime P, Ask K, Kolb M. TGF-β, Smad3 and the process of progressive fibrosis. Biochem Soc Trans. 2007;35:661–4.
Harrington JR. The role of MCP-1 in atherosclerosis. Stem cells. 2000;18:65–6.
Drost J, Clevers H. Organoids in cancer research. Nat Rev Cancer. 2018;18:407–18.
Fatehullah A, Tan SH, Barker N. Organoids as an in vitro model of human development and disease. Nat Cell Biol. 2016;18:246–54.
Ringuette-Goulet C, Bolduc S, Pouliot F. Modeling human bladder cancer. World J Urol. 2018;36:1759–66.
Sachs N, Clevers H. Organoid cultures for the analysis of cancer phenotypes. Curr Opin Genet Dev. 2014;24:68–73.
Tu J, Luo X, Liu H, Zhang J, He M. Cancer spheroids derived exosomes reveal more molecular features relevant to progressed cancer. Biochem Biophys Rep. 2021;26:101026.
Ghafouri-Fard S, Khoshbakht T, Hussen BM, Taheri M, Samadian M. a review on the role of miR-1246 in the pathoetiology of different cancers. Front Mol Biosci. 2022;8:771835.
Pfeffer SR, Yang CH, Pfeffer LM. The role of miR-21 in cancer. Drug Dev Res. 2015;76:270–7.
Wu Q, Luo G, Yang Z, Zhu F, An Y, Shi Y, et al. miR-17-5p promotes proliferation by targeting SOCS6 in gastric cancer cells. FEBS Lett. 2014;588:2055–62.
Sun M, Liu XH, Li JH, Yang JS, Zhang EB, Yin DD, et al. MiR-196a is upregulated in gastric cancer and promotes cell proliferation by downregulating p27kip1. Mol Cancer Ther. 2012;11:842–52.
Wang H, Hu H, Luo Z, Liu S, Wu W, Zhu M, et al. miR-4454 up-regulated by HPV16 E6/E7 promotes invasion and migration by targeting ABHD2/NUDT21 in cervical cancer. Biosci Rep. 2020;40:BSR20200796.
Naseri M, Zöller M, Hadjati J, Ghods R, Ranaei Pirmardan E, Kiani J, et al. Dendritic cells loaded with exosomes derived from cancer stem cell-enriched spheroids as a potential immunotherapeutic option. J Cell Mol Med. 2021;25:3312–26.
Ghosh S, Garg S, Ghosh S. Cell-derived exosome therapy: a novel approach to treat post-traumatic brain injury mediated neural injury. ACS Chem Neurosci. 2020;11:2045–7.
Zhu M, Li Y, Shi J, Feng W, Nie G, Zhao Y. Exosomes as extrapulmonary signaling conveyors for nanoparticle-induced systemic immune activation. Small. 2012;8:404–12.
Diaz-Armas GG, Cervantes-Gonzalez AP, Martinez-Duarte R, Perez-Gonzalez VH. Electrically driven microfluidic platforms for exosome manipulation and characterization. Electrophoresis. 2022;43:327–39.
Acknowledgements
This research was supported by the National Research Foundation of Korea (NRF), and the Ministry of Science and ICT (NRF-2019R1C1C1007384, NRF-2020M2D9A3094171, and NRF-2021R1A4A1032782). This research was also supported by a Korean Fund for Regenerative Medicine (KFRM) grant funded by the Korea government (the Ministry of Science and ICT, the Ministry of Health & Welfare; project Number: 21A0102L1-12). Industrial Technology Alchemist Program funded by Ministry of Trade, Industry, and Energy (MOTIE) of Korea (20018560)
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no financial conflicts of interest.
Ethical statement
No experiments involving animals or humans were conducted in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, DH., Yun, D.W., Kim, Y.H. et al. Various Three-Dimensional Culture Methods and Cell Types for Exosome Production. Tissue Eng Regen Med 20, 621–635 (2023). https://doi.org/10.1007/s13770-023-00551-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-023-00551-y