Abstract
Background:
Biodegradable poly (l-lactic acid) (PLLA), a bio safe polymer with a large elastic modulus, is widely used in biodegradable medical devices. However, because of its poor mechanical properties, a PLLA strut must be made twice as thick as a metal strut for adequate blood vessel support. Therefore, the mechanical properties of a drug-eluting metal-based stents (MBS) and a bioresorbable vascular scaffolds (BVS) were evaluated and their safety and efficacy were examined via a long-term rabbit iliac artery model.
Methods:
The surface morphologies of the MBSs and BVSs were investigated via optical and scanning electron microscopy. An everolimus-eluting (EE) BVS or an EE-MBS was implanted into rabbit iliac arteries at a 1.1:1 stent-to-artery ratio. Twelve months afterward, stented iliac arteries from each group were analyzed via X-ray angiography, optical coherence tomography (OCT), and histopathologic evaluation.
Results:
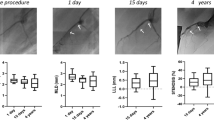
Surface morphology analysis of the EE coating on the MBS confirmed that it was uniform and very thin (4.7 μm). Comparison of the mechanical properties of the EE-MBS and EE-BVS showed that the latter outperformed the former in all aspects (radial force (2.75 vs. 0.162 N/mm), foreshortening (0.24% vs. 1.9%), flexibility (0.52 vs. 0.19 N), and recoil (3.2% vs. 6.3%). At all time points, the percent area restenosis was increased in the EE-BVS group compared to the EE-MBS group. The OCT and histopathological analyses indicate no significant changes in strut thickness.
Conclusion:
BVSs with thinner struts and shorter resorption times should be developed. A comparable long-term safety/efficacy evaluation after complete absorption of BVSs should be conducted.










Similar content being viewed by others
References
Sahin B, Ilgun G. Risk factors of deaths related to cardiovascular diseases in World Health Organization (WHO) member countries. Health Soc Care Community. 2022;30:73–80.
Thygesen K, Alpert JS, White HD, Jaffe AS, Apple FS, Galvani M, et al. Universal definition of myocardial infarction. Circulation. 2007;116:2634–53.
Reimer KA, Jennings RB, Tatum AH. Pathobiology of acute myocardial ischemia: metabolic, functional and ultrastructural studies. Am J Cardiol. 1983;52:72A-81.
Ahmed K, Jeong MH, Chakraborty R, Hong YJ, Sim DS, Hwang SH, et al. Percutaneous coronary intervention with drug-eluting stent implantation vs. coronary artery bypass grafting for multivessel coronary artery disease in metabolic syndrome patients with acute myocardial infarction. Circ J. 2012;76:721–8.
Jeong MH. Current status of the development of new-drug eluting stents. Korean J Med. 2009;76:544–8.
Gruntzig A. Transluminal dilatation of coronary-artery stenosis. Lancet. 1978;1:263.
Sim DS, Jeong MH. Development of novel drug-eluting stents for acute myocardial infarction. Chonnam Med J. 2017;53:187–95.
Joner M, Finn AV, Farb A, Mont EK, Kolodgie FD, Ladich E, et al. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006;48:193–202.
Zamiri P, Kuang Y, Sharma U, Ng TF, Busold RH, Rago AP, et al. The biocompatibility of rapidly degrading polymeric stents in porcine carotid arteries. Biomaterials. 2010;31:7847–55.
Peng HY, Chen M, Zheng B, Wang XG, Huo Y. Long-term effects of novel biodegradable, polymer-coated, sirolimus-eluting stents on neointimal formation in a porcine coronary model. Int Heart J. 2009;50:811–22.
Kim MC, Jeong MH, Sim DS, Hong YJ, Kim JH, Ahn Y. Intravascular ultrasound-guided treatment for in-stent restenosis associated with stent fracture in overlapped drug-eluting stents. Chonnam Med J. 2019;55:165–7.
Jinnouchi H, Torii S, Sakamoto A, Kolodgie FD, Virmani R, Finn AV. Fully bioresorbable vascular scaffolds: lessons learned and future directions. Nat Rev Cardiol. 2019;16:286–304.
Zhang ZQ, Yang YX, Li JA, Zeng RC, Guan SK. Advances in coatings on magnesium alloys for cardiovascular stents - a review. Bioact Mater. 2021;6:4729–57.
Park SA, Lee SJ, Lim KS, Bae IH, Lee JH, Kim WD, et al. In vivo evaluation and characterization of a bio-absorbable drug-coated stent fabricated using a 3D-printing system. Mater Lett. 2015;141:355–8.
Chevalier B, Abizaid A, Carrie D, Frey N, Lutz M, Weber-Albers J, et al. Clinical and angiographic outcomes with a novel radiopaque sirolimus-eluting bioresorbable vascular scaffold. Circ Cardiovasc Interv. 2019;12:e007283.
Jezewski MP, Kubisa MJ, Eyileten C, De Rosa S, Christ G, Lesiak M, et al. Bioresorbable vascular scaffolds-dead end or still a rough diamond. J Clin Med. 2019;8:2167.
Bae IH, Lim KS, Park JK, Park DS, Lee SY, Jang EJ, et al. Mechanical behavior and in vivo properties of newly designed bare metal stent for enhanced flexibility. J Ind Eng Chem. 2015;21:1295–300.
Oh S, Jeong MH, Park DS, Kim M, Kim JH, Hyun DY, et al. Successful implantation of a novel polymer-free everolimus-eluting stent using nitrogen-doped titanium dioxide film with good patency on follow-up angiography: a case report. Medicine (Baltimore). 2022;101:e29666.
Schwartz RS, Huber KC, Murphy JG, Edwards WD, Camrud AR, Vlietstra RE, et al. Restenosis and the proportional neointimal response to coronary artery injury: results in a porcine model. J Am Coll Cardiol. 1992;19:267–74.
Schwartz RS, Edelman E, Virmani R, Carter A, Granada JF, Kaluza GL, et al. Drug-eluting stents in preclinical studies: updated consensus recommendations for preclinical evaluation. Circ Cardiovasc Interv. 2008;1:143–53.
Iqbal J, Gunn J, Serruys PW. Coronary stents: historical development, current status and future directions. Br Med Bull. 2013;106:193–211.
Kee WJ, Jeong MH, Jang SY, Lee MG, Park KH, Sim DS, et al. Very late stent thrombosis due to neointimal rupture after paclitaxel-eluting stent implantation. Korean Circ J. 2011;41:754–8.
Kim Y, Bae S, Jeong MH, Ahn Y, Kim CJ, Cho MC, et al. One-year efficacy and safety of everolimus-eluting bioresorbable scaffolds in the setting of acute myocardial infarction. PLoS One. 2020;15:e0235673.
Kim HI, Ishihara K, Lee S, Seo JH, Kim HY, Suh D, et al. Tissue response to poly(L-lactic acid)-based blend with phospholipid polymer for biodegradable cardiovascular stents. Biomaterials. 2011;32:2241–7.
McDonald RA, Halliday CA, Miller AM, Diver LA, Dakin RS, Montgomery J, et al. reducing in-stent restenosis: therapeutic manipulation of miRNA in vascular remodeling and inflammation. J Am Coll Cardiol. 2015;65:2314–27.
Nebeker JR, Virmani R, Bennett CL, Hoffman JM, Samore MH, Alvarez J, et al. Hypersensitivity cases associated with drug-eluting coronary stents: a review of available cases from the research on adverse drug events and reports (RADAR) project. J Am Coll Cardiol. 2006;47:175–81.
Sanchez-Fructuoso AI. Everolimus: an update on the mechanism of action, pharmacokinetics and recent clinical trials. Expert Opin Drug Metab Toxicol. 2008;4:807–19.
Park DS, Bae IH, Jeong MH, Lim KS, Sim DS, Hong YJ, et al. In vitro and in vivo evaluation of a novel polymer-free everolimus-eluting stent by nitrogen-doped titanium dioxide film deposition. Mater Sci Eng C Mater Biol Appl. 2018;91:615–23.
Acknowledgements
This work was supported by a Korean Medical Device Development Fund Grant funded by the Korean government (the Ministries of Science and ICT; Trade, Industry, and Energy; Health and Welfare; and Food and Drug Safety) (1711138916, KMDF_PR_20200901_0280 and 1711137864,KMDF_PR_20200901_0005). This research was also supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Korean Ministry of Education (NRF-2020R1I1A3A04036675).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing financial interests.
Ethical statement
The animal studies were performed after receiving approval from the Institutional Animal Care and Use Committee (IACUC) in Chonnam National University Hospital (IACUC approval No. CNUHIACUC-21004).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Park, D.S., Jeong, M.H., Jin, Y.J. et al. Preclinical Evaluation of an Everolimus-Eluting Bioresorbable Vascular Scaffold Via a Long-Term Rabbit Iliac Artery Model. Tissue Eng Regen Med 20, 239–249 (2023). https://doi.org/10.1007/s13770-023-00518-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-023-00518-z