Abstract
Background:
Diabetic wound healing remains a major challenge due to the impaired functionality of angiogenesis by persistent hyperglycemia. Mesenchymal stem cell exosomes are appropriate candidates for regulating the formation of angiogenesis in tissue repair and regeneration. Here, we explored the effects of exosomes derived from human amniotic mesenchymal stem cell (hAMSC-Exos) on the biological activities of human umbilical vein endothelial cells (HUVECs) treated with high glucose and on diabetic wound healing and investigate lncRNAs related to angiogenesis in hAMSC-Exos.
Methods:
hAMSCs and hAMSC-Exos were isolated and identified by flow cytometry or western blot. A series of functional assays such as cell counting kit-8, scratching, transwell and tube formation assays were performed to evaluate the potential effect of hAMSC-Exos on high glucose-treated HUVECs. The effect of hAMSC-Exos on diabetic wound healing were tested by measuring wound closure rates and immunohistochemical staining of CD31. Subsequently, the lncRNAs profiles in hAMSC-Exos and hAMSCs were examined to screen the lncRNAs related to angiogenesis.
Results:
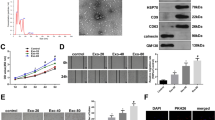
The isolated hAMSC-Exos had a size range of 30–150 nm and were positive for CD9, CD63 and CD81. The hAMSC-Exos facilitate the functional properties of high glucose-treated HUVECs including the proliferation, migration and the angiogenic activities as well as wound closure and angiogenesis in diabetic wound. hAMSC-Exos were enriched lncRNAs that related to angiogenesis, including PANTR1, H19, OIP5-AS1 and NR2F1-AS1.
Conclusion:
Our findings demonstrated hAMSC-Exos facilitate diabetic wound healing by angiogenesis and contain several exosomal lncRNAs related to angiogenesis, which may represent a promising strategy for diabetic wound healing.








Similar content being viewed by others
References
An T, Chen Y, Tu Y, Lin P. Mesenchymal stromal cell-derived extracellular vesicles in the treatment of diabetic foot ulcers: application and challenges. Stem Cell Rev Rep. 2021;17:369–78.
Burgess JL, Wyant WA, Abdo AB, Kirsner RS, Jozic I. Diabetic wound-healing science. Medicina (Kaunas). 2021;57:1072.
Antoniadou E, David AL. Placental stem cells. Best Pract Res Clin Obstet Gynaecol. 2016;31:13–29.
Abbasi-Kangevari M, Ghamari SH, Safaeinejad F, Bahrami S, Niknejad H. Potential therapeutic features of human amniotic mesenchymal stem cells in multiple sclerosis: immunomodulation, inflammation suppression, angiogenesis promotion, oxidative stress inhibition, neurogenesis induction, mmps regulation, and remyelination stimulation. Front Immunol. 2019;10:238.
Parolini O, Alviano F, Bagnara GP, Bilic G, Buhring HJ, Evangelista M, et al. Concise review: isolation and characterization of cells from human term placenta: outcome of the first international workshop on placenta derived stem cells. Stem Cells. 2008;26:300–11.
Azari Z, Nazarnezhad S, Webster TJ, Hoseini SJ, Brouki Milan P, Baino F, et al. Stem cell-mediated angiogenesis in skin tissue engineering and wound healing. Wound Repair Regen. 2022;30:421–35.
Mankuzhy PD, Ramesh ST, Thirupathi Y, Mohandas PS, Chandra V, Sharma TG. The preclinical and clinical implications of fetal adnexa derived mesenchymal stromal cells in wound healing therapy. Wound Repair Regen. 2021;29:347–69.
Yu M, Liu W, Li J, Lu J, Lu H, Jia W, et al. Exosomes derived from atorvastatin-pretreated MSC accelerate diabetic wound repair by enhancing angiogenesis via AKT/eNOS pathway. Stem Cell Res Ther. 2020;11:350.
Yan C, Xv Y, Lin Z, Endo Y, Xue H, Hu Y, et al. Human umbilical cord mesenchymal stem cell-derived exosomes accelerate diabetic wound healing via ameliorating oxidative stress and promoting angiogenesis. Front Bioeng Biotechnol. 2022;10: 829868.
Wang L, Cai Y, Zhang Q, Zhang Y. Pharmaceutical activation of Nrf2 accelerates diabetic wound healing by exosomes from bone marrow mesenchymal stem cells. Int J Stem Cells. 2022;15:164–72.
Ha DH, Kim HK, Lee J, Kwon HH, Park GH, Yang SH, et al. Mesenchymal stem/stromal cell-derived exosomes for immunomodulatory therapeutics and skin regeneration. Cells. 2020;9:1157.
Casado-Diaz A, Quesada-Gomez JM, Dorado G. Extracellular vesicles derived from mesenchymal stem cells (MSC) in regenerative medicine: applications in skin wound healing. Front Bioeng Biotechnol. 2020;8:146.
Han ZF, Cao JH, Liu ZY, Yang Z, Qi RX, Xu HL. Exosomal lncRNA KLF3-AS1 derived from bone marrow mesenchymal stem cells stimulates angiogenesis to promote diabetic cutaneous wound healing. Diabetes Res Clin Pract. 2022;183:109126.
Born LJ, Chang KH, Shoureshi P, Lay F, Bengali S, Hsu A, et al. HOTAIR-loaded mesenchymal stem/stromal cell extracellular vesicles enhance angiogenesis and wound healing. Adv Healthc Mater. 2022;11:e2002070.
Liu QW, Liu QY, Li JY, Wei L, Ren KK, Zhang XC, et al. Therapeutic efficiency of human amniotic epithelial stem cell-derived functional hepatocyte-like cells in mice with acute hepatic failure. Stem Cell Res Ther. 2018;9:321.
Purushothaman A. Exosomes from cell culture-conditioned medium: isolation by ultracentrifugation and characterization. Methods Mol Biol. 2019;1952:233–44.
Adnan M, Morton G, Hadi S. Analysis of rpoS and bolA gene expression under various stress-induced environments in planktonic and biofilm phase using 2(-ΔΔCT) method. Mol Cell Biochem. 2011;357:275–82.
Chu J, Shi P, Deng X, Jin Y, Liu H, Chen M, et al. Dynamic multiphoton imaging of acellular dermal matrix scaffolds seeded with mesenchymal stem cells in diabetic wound healing. J Biophotonics. 2018;11:e201700336.
Guo J, Hu H, Gorecka J, Bai H, He H, Assi R, et al. Adipose-derived mesenchymal stem cells accelerate diabetic wound healing in a similar fashion as bone marrow-derived cells. Am J Physiol Cell Physiol. 2018;315:C885–96.
Kim SW, Zhang HZ, Guo L, Kim JM, Kim MH. Amniotic mesenchymal stem cells enhance wound healing in diabetic NOD/SCID mice through high angiogenic and engraftment capabilities. PLoS One. 2012;7:e41105.
Liu WZ, Ma ZJ, Li JR, Kang XW. Mesenchymal stem cell-derived exosomes: therapeutic opportunities and challenges for spinal cord injury. Stem Cell Res Ther. 2021;12:102.
Shabbir A, Cox A, Rodriguez-Menocal L, Salgado M, Van Badiavas E. Mesenchymal stem cell exosomes induce proliferation and migration of normal and chronic wound fibroblasts, and enhance angiogenesis in vitro. Stem Cells Dev. 2015;24:1635–47.
Tang Y, Zhou Y, Li HJ. Advances in mesenchymal stem cell exosomes: a review. Stem Cell Res Ther. 2021;12:71.
Xiong J, Hu H, Guo R, Wang H, Jiang H. Mesenchymal stem cell exosomes as a new strategy for the treatment of diabetes complications. Front Endocrinol (Lausanne). 2021;12:646233.
Xunian Z, Kalluri R. Biology and therapeutic potential of mesenchymal stem cell-derived exosomes. Cancer Sci. 2020;111:3100–10.
Li X, Xie X, Lian W, Shi R, Han S, Zhang H, et al. Exosomes from adipose-derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model. Exp Mol Med. 2018;50:1–14.
Teng L, Maqsood M, Zhu M, Zhou Y, Kang M, Zhou J, Chen J. Exosomes derived from human umbilical cord mesenchymal stem cells accelerate diabetic wound healing via promoting M2 macrophage polarization, angiogenesis, and collagen deposition. Int J Mol Sci. 2022;23:10421.
Takahashi H, Ohnishi S, Yamamoto Y, Hayashi T, Murao N, Osawa M, et al. Topical application of conditioned medium from hypoxically cultured amnion-derived mesenchymal stem cells promotes wound healing in diabetic mice. Plast Reconstr Surg. 2021;147:1342–52.
Li KS, Bai Y, Li J, Li SL, Pan J, Cheng YQ, et al. LncRNA HCP5 in hBMSC-derived exosomes alleviates myocardial ischemia reperfusion injury by sponging miR-497 to activate IGF1/PI3K/AKT pathway. Int J Cardiol. 2021;342:72–81.
Su Y, Liu Y, Ma C, Guan C, Ma X, Meng S. Mesenchymal stem cell-originated exosomal lncRNA HAND2-AS1 impairs rheumatoid arthritis fibroblast-like synoviocyte activation through miR-143-3p/TNFAIP3/NF-kappaB pathway. J Orthop Surg Res. 2021;16:116.
Liu Y, Lin L, Zou R, Wen C, Wang Z, Lin F. MSC-derived exosomes promote proliferation and inhibit apoptosis of chondrocytes via lncRNA-KLF3-AS1/miR-206/GIT1 axis in osteoarthritis. Cell Cycle. 2018;17:2411–22.
Mao Q, Liang XL, Zhang CL, Pang YH, Lu YX. LncRNA KLF3-AS1 in human mesenchymal stem cell-derived exosomes ameliorates pyroptosis of cardiomyocytes and myocardial infarction through miR-138-5p/Sirt1 axis. Stem Cell Res Ther. 2019;10:393.
Seles M, Hutterer GC, Foßelteder J, Svoboda M, Resel M, Barth DA, et al. Long non-coding RNA PANTR1 is associated with poor prognosis and influences angiogenesis and apoptosis in clear-cell renal cell cancer. Cancers (Basel). 2020;12:1200.
Behera J, Kumar A, Voor MJ, Tyagi N. Exosomal lncRNA-H19 promotes osteogenesis and angiogenesis through mediating Angpt1/Tie2-NO signaling in CBS-heterozygous mice. Theranostics. 2021;11:7715–34.
Li Y, Lin S, Xie X, Zhu H, Fan T, Wang S. Highly enriched exosomal lncRNA OIP5-AS1 regulates osteosarcoma tumor angiogenesis and autophagy through miR-153 and ATG5. Am J Transl Res. 2021;13:4211–23.
Zhang Q, Li T, Wang Z, Kuang X, Shao N, Lin Y. lncRNA NR2F1-AS1 promotes breast cancer angiogenesis through activating IGF-1/IGF-1R/ERK pathway. J Cell Mol Med. 2020;24:8236–47.
Lang HL, Hu GW, Chen Y, Liu Y, Tu W, Lu YM, et al. Glioma cells promote angiogenesis through the release of exosomes containing long non-coding RNA POU3F3. Eur Rev Med Pharmacol Sci. 2017;21:959–72.
Shi C, Yang Q, Pan S, Lin X, Xu G, Luo Y, et al. LncRNA OIP5-AS1 promotes cell proliferation and migration and induces angiogenesis via regulating miR-3163/VEGFA in hepatocellular carcinoma. Cancer Biol Ther. 2020;21:604–14.
Yuan Z, Bian Y, Ma X, Tang Z, Chen N, Shen M. LncRNA H19 knockdown in human amniotic mesenchymal stem cells suppresses angiogenesis by associating with EZH2 and activating vasohibin-1. Stem Cells Dev. 2019;28:781–90.
Sun B, Ding Y, Jin X, Xu S, Zhang H. Long non-coding RNA H19 promotes corneal neovascularization by targeting microRNA-29c. Biosci Rep, 2019;39:BSR20182394.
Peng WX, He PX, Liu LJ, Zhu T, Zhong YQ, Xiang L, et al. LncRNA GAS5 activates the HIF1A/VEGF pathway by binding to TAF15 to promote wound healing in diabetic foot ulcers. Lab Invest. 2021;101:1071–83.
Li B, Luan S, Chen J, Zhou Y, Wang T, Li Z, et al. The MSC-derived exosomal lncRNA H19 promotes wound healing in diabetic foot ulcers by upregulating PTEN via MicroRNA-152-3p. Mol Ther Nucleic Acids. 2020;19:814–26.
Hafez YM, El-Deeb OS, Atef MM. The emerging role of the epigenetic enzyme Sirtuin-1 and high mobility group Box 1 in patients with diabetic foot ulceration. Diabetes Metab Syndr. 2018;12:1065–70.
Acknowledgement
This work was supported by the National Natural Science Foundation of China (Grant No. 81460293).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No potential conflict of interest was reported by the authors.
Ethical statement
This study approved by the Medical Research Ethics Committees of the First Affiliated Hospital of Nanchang University (no. 20218-008) and the written informed consents were signed by the pregnant women or their legal guardians.
The animal experiment were approved by the Experimental Animal Welfare Ethics Committee of the First Affiliated Hospital of Nanchang University (Jiangxi, China) (CDYFY-IACUC-202208QR040).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
13770_2022_513_MOESM2_ESM.docx
Supplemental Fig. 1. A Flow cytometry analysis of the CD44, CD73, CD90, CD105, CD34, CD45 and HLA-DR expressions in hAMSCs. Blue lines represent the isotype control, and red lines represent the level of surface markers. B and C The differentiation potential of hAMSCs. The hAMSCs could be induced to differentiate into osteocytes and adipocytes with osteogenic and adipogenic differentiation medium, respectively, and the hAMSCs were stained with Alizarin Red S and Oil Red O after osteocytes and adipocytes differentiated (DOCX 734 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fu, S., Zhang, H., Li, X. et al. Exosomes Derived from Human Amniotic Mesenchymal Stem Cells Facilitate Diabetic Wound Healing by Angiogenesis and Enrich Multiple lncRNAs. Tissue Eng Regen Med 20, 295–308 (2023). https://doi.org/10.1007/s13770-022-00513-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-022-00513-w