Abstract
Background:
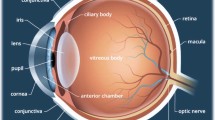
Eye irritation tests with animals have been conducted for a long time. However, the subjective decision to irritation, the anatomic/physiologic difference between species and humans, and ethical issues are crucial problems. Various research groups have paid attention to alternative testing methods. In these senses, we fabricated in vitro mini-cornea models with immortalized human corneal epithelial cells (iHCECs) and keratocytes (iHCKs) and used them for irritation tests. This study hypothesized that our mini-cornea model could present different viability tendencies according to test chemicals with different irritancy levels.
METHODS:
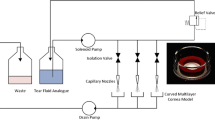
Cells used in this study were characterized with cornea-specific markers by immunocytochemistry and western blot. To make a three-dimensional hemisphere construct like cornea stroma, we cultured iHCKs under modified culture conditions verified by matrix formation and total collagen content. iHCECs were seeded on the construct and cultured at an air–liquid interface. The model was treated with 2-phenoxyethanol, triton X-100, sodium lauryl sulfate, and benzalkonium chloride.
RESULTS:
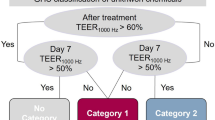
iHCECs and iHCKs presented their specific cell markers. In modifying the culture condition, the group treating ascorbic acid (200 µg/ml) presented an intact cellular matrix and included the highest collagen content; thus, we used this condition to fabricate the mini-cornea model. The model shows hemisphere shape and homogenous cell distributions in histological analysis. We observed different sensitivity tendencies by types of chemicals, and the model's viability significantly decreased when the chemical concentration increased.
CONCLUSION:
In this study, we performed and observed irritation tests using a tissue-engineered mini-cornea model and considered to apply as an alternative approach for animal tests.






Similar content being viewed by others
References
Abou Shousha M, Wang J, Kontadakis G, Feuer W, Canto AP, Hoffmann R, et al. Corneal epithelial thickness profile in dry-eye disease. Eye (Lond). 2020;34:915–22.
DelMonte DW, Kim T. Anatomy and physiology of the cornea. J Cataract Refract Surg. 2011;37:588–98.
Lagali N. Corneal stromal regeneration: current status and future therapeutic potential. Curr Eye Res. 2020;45:278–90.
Syed-Picard FN, Du Y, Hertsenberg AJ, Palchesko R, Funderburgh ML, Feinberg AW, et al. Scaffold-free tissue engineering of functional corneal stromal tissue. J Tissue Eng Regen Med 2018; 12:59–69.
Sridhar MS. Anatomy of cornea and ocular surface. Indian J Ophthalmol. 2018;66:190–4.
York M, Steiling W. A critical review of the assessment of eye irritation potential using the Draize rabbit eye test. J Appl Toxicol. 1998;18:233–40.
Wilhelmus KR. The Draize eye test. Surv Ophthalmol. 2001;45:493–515.
Chan T, Payor S, Holden BA. Corneal thickness profiles in rabbits using an ultrasonic pachometer. Invest Ophthalmol Vis Sci. 1983;24:1408–10.
Mishima S, Gasset A, Klyce SD, Baum JL. Determination of tear volume and tear flow. Invest Ophthalmol. 1966;5:264–76.
Whittaker AL, Williams DL. Evaluation of lacrimation characteristics in clinically normal New Zealand white rabbits by using the schirmer tear test I. J Am Assoc Lab Anim Sci. 2015;54:783–7.
de Vries RB, Leenaars M, Tra J, Huijbregtse R, Bongers E, Jansen JA, et al. The potential of tissue engineering for developing alternatives to animal experiments: a systematic review. J Tissue Eng Regen Med. 2015;9:771–8.
Lotz C, Kiesewetter L, Schmid FF, Hansmann J, Walles H, Groeber-Becker F. Replacing the Draize eye test: impedance spectroscopy as a 3R method to discriminate between all GHS categories for eye irritation. Sci Rep. 2018;8:15049.
Jung KM, Lee SH, Ryu YH, Jang WH, Jung HS, Han JH, et al. A new 3D reconstituted human corneal epithelium model as an alternative method for the eye irritation test. Toxicol In Vitro. 2011;25:403–10.
Kolle SN, Rey Moreno MC, Mayer W, van Cott A, van Ravenzwaay B, Landsiedel R. The EpiOcular™ eye irritation test is the method of choice for the in vitro eye irritation testing of agrochemical formulations: correlation analysis of EpiOcular Eye Irritation Test and BCOP Test Data According to the UN GHS, US EPA and Brazil ANVISA Classification Schemes. Altern Lab Anim. 2015;43:181–98.
Stern M, Klausner M, Alvarado R, Renskers K, Dickens M. Evaluation of the EpiOcular™ tissue model as an alternative to the draize eye irritation test. Toxicol In Vitro. 1998;12:455–61.
Lee M, Hwang JH, Lim KM. Alternatives to in vivo draize rabbit eye and skin irritations tests with a focus on 3D reconstructed human cornea-like epithelium and epidermis models. Toxicol Res. 2017;33:191–203.
Katoh M, Hamajima F, Ogasawara T, Hata K. Establishment of a new in vitro test method for evaluation of eye irritancy using a reconstructed human corneal epithelial model. LabCyte CORNEA-MODEL Toxicol In Vitro. 2013;27:2184–92.
Kojima H, Ando Y, Idehara K, Katoh M, Kosaka T, Miyaoka E, et al. Validation study of the in vitro skin irritation test with the LabCyte EPI-MODEL24. Altern Lab Anim. 2012;40:33–50.
Lim SE, Ha SJ, Jang WH, Jung KM, Jung MS, Yeo KW, et al. Me-too validation study for in vitro eye irritation test with 3D-reconstructed human cornea epithelium. MCTT HCE Toxicol In Vitro. 2019;55:173–84.
Nowell CS, Radtke F. Corneal epithelial stem cells and their niche at a glance. J Cell Sci. 2017;130:1021–5.
Meek KM. Corneal collagen-its role in maintaining corneal shape and transparency. Biophys Rev. 2009;1:83–93.
Proulx S, d’Arc Uwamaliya J, Carrier P, Deschambeault A, Audet C, Giasson CJ, et al. Reconstruction of a human cornea by the self-assembly approach of tissue engineering using the three native cell types. Mol Vis. 2010;16:2192–201.
Nishida K, Yamato M, Hayashida Y, Watanabe K, Maeda N, Watanabe H, et al. Functional bioengineered corneal epithelial sheet grafts from corneal stem cells expanded ex vivo on a temperature-responsive cell culture surface. Transplantation. 2004;77:379–85.
Yu J, Tu YK, Tang YB, Cheng NC. Stemness and transdifferentiation of adipose-derived stem cells using L-ascorbic acid 2-phosphate-induced cell sheet formation. Biomaterials. 2014;35:3516–26.
Rosell-Garcia T, Rodriguez-Pascual F. Enhancement of collagen deposition and cross-linking by coupling lysyl oxidase with bone morphogenetic protein-1 and its application in tissue engineering. Sci Rep. 2018;8:10780.
Mertsch S, Hasenzahl M, Reichl S, Geerling G, Schrader S. Decellularized human corneal stromal cell sheet as a novel matrix for ocular surface reconstruction. J Tissue Eng Regen Med. 2020;14:1318–32.
Chen J, Lan J, Liu D, Backman LJ, Zhang W, Zhou Q, et al. Ascorbic acid promotes the stemness of corneal epithelial stem/progenitor cells and accelerates epithelial wound healing in the cornea. Stem Cells Transl Med. 2017;6:1356–65.
Lee M, Joo KM, Choi S, Lee SH, Kim SY, Chun YJ, et al. Nervonoylceramide (C24:1Cer), a lipid biomarker for ocular irritants released from the 3D reconstructed human cornea-like epithelium, MCTT HCE™. Toxicol In Vitro. 2018;47:94–102.
Merjava S, Neuwirth A, Tanzerova M, Jirsova K. The spectrum of cytokeratins expressed in the adult human cornea, limbus and perilimbal conjunctiva. Histol Histopathol. 2011;26:323–31.
Auw-Haedrich C, Agrawal M, Gabbert HE, Meyer P, Arnold N, Reinhard T. Immunohistochemical expression of epithelial cell markers in corneas with congenital aniridia and ocular cicatrizing pemphigoid. Acta Ophthalmol. 2011;89:47–53.
Jonas JB, Holbach L. Central corneal thickness and thickness of the lamina cribrosa in human eyes. Invest Ophthalmol Vis Sci. 2005;46:1275–9.
Offord EA, Sharif NA, Macé K, Tromvoukis Y, Spillare EA, Avanti O, et al. Immortalized human corneal epithelial cells for ocular toxicity and inflammation studies. Invest Ophthalmol Vis Sci. 1999;40:1091–101.
Robin JB, Schanzlin DJ, Verity SM, Barron BA, Arffa RC, Suarez E, et al. Peripheral corneal disorders. Surv Ophthalmol. 1986;31:1–36.
Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci. 2010;123:4195–200.
Steiling W, Bracher M, Courtellemont P, de Silva O. The HET-CAM, a useful in vitro assay for assessing the eye irritation properties of cosmetic formulations and ingredients. Toxicol In Vitro. 1999;13:375–84.
Dréno B, Zuberbier T, Gelmetti C, Gontijo G, Marinovich M. Safety review of phenoxyethanol when used as a preservative in cosmetics. J Eur Acad Dermatol Venereol. 2019;33:15–24.
Koley D, Bard AJ. Triton X-100 concentration effects on membrane permeability of a single HeLa cell by scanning electrochemical microscopy (SECM). Proc Natl Acad Sci U S A. 2010;107:16783–7.
Choi S, Lee M, Lee SH, Jung HS, Kim SY, Chung TY, et al. Identification of cornifelin and early growth response-1 gene as novel biomarkers for in vitro eye irritation using a 3D reconstructed human cornea model MCTT HCE™. Arch Toxicol. 2015;89:1589–98.
Kusuma SAF, Abdassah M, Maryati F. Comparison of perservatives efficacy of benzalkonium chloride, thimerosal, and benzyl alcohol in eye drop products containing chloramphenicol. Int J Appl Pharm. 2020;12:100–5.
Acknowledgements
This research was supported by Grant from the National Research Foundation of Korea (NRF-2019M3E5D1A02070861 and NRF-2022R1A2C1091873).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no conflicts of interest.
Ethical statement
There are no animal experiments carried out for this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, SH., Jo, SH., Kim, B.K. et al. Tissue Engineered Mini-Cornea Model for Eye Irritation Test. Tissue Eng Regen Med 20, 213–223 (2023). https://doi.org/10.1007/s13770-022-00504-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-022-00504-x