Abstract
Background:
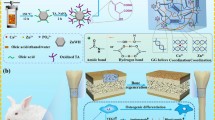
Due to the increasing aging of society, the number of patients suffering from senile diseases is increasing. Patients suffering from osteoporosis, which is a representative senile disease, take a long time to recover from fractures, and the resulting mortality rate is very high. Alendronate (Ald), which is widely used as a treatment for osteoporosis, alleviates osteoporosis by inhibiting osteoclasts. In addition, whitlockite (WH) promotes the osteogenic differentiation of bone cells and improves bone regeneration. Therefore, we intended to bring about a synergistic effect by using these substances together.
Methods:
In this study, a scaffold composed of gelatin/heparin was fabricated and applied to effectively use WH and Ald together. A scaffold was constructed using gelatin and heparin was used to effectively utilize the cations released from WH. In addition, it formed a porous structure for effective bone regeneration. In vitro and in vivo osteoclast inhibition, osteogenic differentiation, and bone regeneration were studied using the prepared scaffolds.
Results:
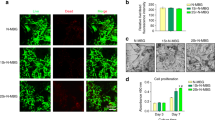
The inhibition of osteoclast was much higher when WH and Ald were applied in combination rather than individually. The highest level of osteogenic differentiation was observed when both substances were applied simultaneously. In addition, when applied to bone regeneration through the mouse calvarial defect model, combined treatment showed excellent bone regeneration.
Conclusion:
Therefore, this study showed the synergistic effect of WH and Ald, and it is suggested that better bone regeneration is possible by applying this treatment to bones with fractures that are difficult to regenerate.





Similar content being viewed by others
References
Bliuc D, Nguyen ND, Milch VE, Nguyen TV, Eisman JA, Center JR. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA. 2009;301:513–21.
Padilla Colón CJ, Molina-Vicenty IL, Frontera-Rodríguez M, García-Ferré A, Rivera BP, Cintrón-Vélez G, et al. Muscle and bone mass loss in the elderly population: advances in diagnosis and treatment. J Biomed (Syd). 2018;3:40–9.
Li G, Thabane L, Papaioannou A, Ioannidis G, Levine MA, Adachi JD. An overview of osteoporosis and frailty in the elderly. BMC Musculoskelet Disord. 2017;18:46.
Vandenbroucke A, Luyten FP, Flamaing J, Gielen E. Pharmacological treatment of osteoporosis in the oldest old. Clin Interv Aging. 2017;12:1065–77.
Lin JT, Lane JM. Osteoporosis: a review. Clin Orthop Relat Res. 2004;425:126–34.
Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet. 2011;377:1276–87.
Tu KN, Lie JD, Wan CKV, Cameron M, Austel AG, Nguyen JK, et al. Osteoporosis: a review of treatment options. P T. 2018;43:92–104.
Blume SW, Curtis JR. Medical costs of osteoporosis in the elderly Medicare population. Osteoporos Int. 2011;22:1835–44.
Cheung CL, Ang SB, Chadha M, Chow ES, Chung YS, Hew FL, et al. An updated hip fracture projection in Asia: the Asian federation of osteoporosis societies study. Osteoporos Sarcopenia. 2018;4:16–21.
Ensrud KE, Ewing SK, Taylor BC, Fink HA, Stone KL, Cauley JA, et al. Frailty and risk of falls, fracture, and mortality in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci. 2007;62:744-51.
Mears DC. Surgical treatment of acetabular fractures in elderly patients with osteoporotic bone. J Am Acad Orthop Surg. 1999;7:128–41.
Mohd-Tahir NA, Li SC. Economic burden of osteoporosis-related hip fracture in Asia: a systematic review. Osteoporos Int. 2017;28:2035–44.
Kannus P, Parkkari J, Koskinen S, Niemi S, Palvanen M, Järvinen M, et al. Fall-induced injuries and deaths among older adults. JAMA. 1999;281:1895–9.
McCombs JS, Thiebaud P, McLaughlin-Miley C, Shi J. Compliance with drug therapies for the treatment and prevention of osteoporosis. Maturitas. 2004;48:271–87.
Saag KG, Emkey R, Schnitzer TJ, Brown JP, Hawkins F, Goemaere S, et al. Alendronate for the prevention and treatment of glucocorticoid-induced osteoporosis. N Engl J Med. 1998;339:292–9.
Cummings SR, Santora AC, Black DM, Russell RGG. History of alendronate. Bone. 2020;137:115411.
Bone HG, Hosking D, Devogelaer JP, Tucci JR, Emkey RD, Tonino RP, et al. Ten years’ experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med. 2004;350:1189–99.
Orwoll E, Ettinger M, Weiss S, Miller P, Kendler D, Graham J, et al. Alendronate for the treatment of osteoporosis in men. N Engl J Med. 2000;343:604–10.
Saag KG, Petersen J, Brandi ML, Karaplis AC, Lorentzon M, Thomas T, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. 2017;377:1417–27.
Fu L, Tang T, Miao Y, Zhang S, Qu Z, Dai K. Stimulation of osteogenic differentiation and inhibition of adipogenic differentiation in bone marrow stromal cells by alendronate via ERK and JNK activation. Bone. 2008;43:40–7.
Kim HK, Kim JH, Abbas AA, Yoon TR. Alendronate enhances osteogenic differentiation of bone marrow stromal cells: a preliminary study. Clin Orthop Relat Res. 2009;467:3121–8.
Xiong Y, Yang HJ, Feng J, Shi ZL, Wu LD. Effects of alendronate on the proliferation and osteogenic differentiation of MG-63 cells. J Int Med Res. 2009;37:407–16.
Jeong J, Kim JH, Shim JH, Hwang NS, Heo CY. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater Res. 2019;23:4.
Jang HL, Jin K, Lee J, Kim Y, Nahm SH, Hong KS, et al. Revisiting whitlockite, the second most abundant biomineral in bone: nanocrystal synthesis in physiologically relevant conditions and biocompatibility evaluation. ACS Nano. 2014;8:634–41.
Jang HL, Zheng GB, Park J, Kim HD, Baek HR, Lee HK, et al. In vitro and in vivo evaluation of whitlockite biocompatibility: comparative study with hydroxyapatite and β-tricalcium phosphate. Adv Healthc Mater. 2016;5:128–36.
Kim HD, Jang HL, Ahn HY, Lee HK, Park J, Lee ES, et al. Biomimetic whitlockite inorganic nanoparticles-mediated in situ remodeling and rapid bone regeneration. Biomaterials. 2017;112:31–43.
Kuo ZK, Lai PL, Toh EK, Weng CH, Tseng HW, Chang PZ, et al. Osteogenic differentiation of preosteoblasts on a hemostatic gelatin sponge. Sci Rep. 2016;6:32884.
Nair M, Nancy D, Krishnan AG, Anjusree GS, Vadukumpully S, Nair SV, et al. Graphene oxide nanoflakes incorporated gelatin–hydroxyapatite scaffolds enhance osteogenic differentiation of human mesenchymal stem cells. Nanotechnology. 2015;26:161001.
Takahashi Y, Yamamoto M, Tabata Y. Osteogenic differentiation of mesenchymal stem cells in biodegradable sponges composed of gelatin and β-tricalcium phosphate. Biomaterials. 2005;26:3587–96.
Singh AK, Bhadauria AS, Kumar P, Bera H, Saha S, et al. Bioactive and drug-delivery potentials of polysaccharides and their derivatives. In: Polysaccharide Carriers for Drug Delivery. Woodhead Publishing, 2019. p. 19–48.
Liu C, Lin C, Feng X, Wu Z, Lin G, Quan C, et al. A biomimicking polymeric cryogel scaffold for repair of critical-sized cranial defect in a rat model. Tissue Eng Part A. 2019;25:1591–604.
Shalumon KT, Liao HT, Kuo CY, Wong CB, Li CJ, P A M, et al. Rational design of gelatin/nanohydroxyapatite cryogel scaffolds for bone regeneration by introducing chemical and physical cues to enhance osteogenesis of bone marrow mesenchymal stem cells. Mater Sci Eng C Mater Biol Appl. 2019;104:109855.
Tsung LH, Chang KH, Chen JP. Osteogenesis of adipose-derived stem cells on three-dimensional, macroporous gelatin–hyaluronic acid cryogels. Biomed Eng (Singapore). 2011;23:127–33.
Stevic I, Parmar N, Paredes N, Berry LR, Chan AK. Binding of heparin to metals. Cell Biochem Biophys. 2011;59:171–8.
Al Deeb SK, Hamdan II, Al Najjar SM. Spectroscopic and HPLC methods for the determination of alendronate in tablets and urine. Talanta. 2004;64:695–702.
Acknowledgements
This work was supported by Seoul National University Research Grant in “2017”.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical statement
Human adipose-derived stem cells were purchased from Lonza (PT-5006, Lot.0000605220). The animal studies were performed after receiving approval from the Institutional Animal Care and Use Committee (IACUC) of Seoul National University Bundang Hospital (IACUC approval No. BA-2103–315-019–01).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jeong, J., Shim, J.H., Koo, B.M. et al. Synergistic Effect of Whitlockite Scaffolds Combined with Alendronate to Promote Bone Regeneration. Tissue Eng Regen Med 19, 83–92 (2022). https://doi.org/10.1007/s13770-021-00416-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-021-00416-2