Abstract
Background:
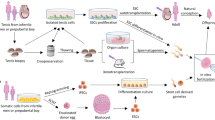
To investigate the in vitro induction of salivary gland-like tissue by ips cells in an interferon regulatory factor 6 (IRF6) overexpression and parotid conditioned medium environment.
Methods:
Urine-derived ips cells were isolated, identified, transfected with IRF6 and cultured in parotid conditioned medium to induce ips cells into salivary gland differentiation, morphological changes of ips cells were observed, CCK-8 was used to determine the cell proliferation efficiency and transcriptome sequencing was used to detect the expression of genes related to parotid gland formation.
Results:
Immunofluorescence staining showed that the isolated ips cells were positive for NANOG, SSEA4 and OCT4 and had embryonic-like stem cell characteristics; CCK-8 showed that there was no statistical difference in the proliferation efficiency between the IRF6+ induced group and the simple induced group after induction of ips cells into salivary glands. The results of transcriptome sequencing showed that there were a total of 643 differentially expressed genes, including 365 up-regulated genes and 278 down-regulated genes in the IRF6+ induced group compared to the blank control group, and the salivary gland related genes HAPLN1, CCL2, MSX2, ANXA1, CYP11A1, HES1 and LUM were all highly expressed in the IRF6+ induced group.
Conclusion:
IRF6 promotes salivary gland differentiation in urine-derived iPSCs, and its mechanism of promoting differentiation may be that IRF6 upregulates the expression of HAPLN1, CCL2, MSX2, ANXA1, CYP11A1, HES1 and LUM to promote epithelial differentiation.













Similar content being viewed by others
References
Ysik D, Niemirowicz-Laskowska K, Bucki R, Tokajuk G, Mystkowska J. Artificial saliva: challenges and future perspectives for the treatment of xerostomia. Int J Mol Sci. 2019;20:3199.
Mitroulia A, Gavriiloglou M, Athanasiadou P, Bakopoulou A, Poulopoulos A, Panta P, et al. Salivary gland stem cells and tissue regeneration: an update on possible therapeutic application. J Contemp Dent Pract. 2019;20:978–86.
Rocchi C, Emmerson E. Mouth-watering results: clinical need, current approaches, and future directions for salivary gland regeneration. Trends Mol Med. 2020;26:649–69.
Chhabra A. Derivation of human induced pluripotent stem cell (iPSC) lines and mechanism of pluripotency: historical perspective and recent advances. Stem Cell Rev Rep. 2017;13:757–73.
Moretti F, Marinari B, Lo Iacono N, Botti E, Giunta A, Spallone G, et al. A regulatory feedbackloop involving p63 and IRF6 links the pathogenesis of 2 genetically different human ectodermal dysplasias. J Clin Invest. 2010;120:1570–7.
Fakhouri WD, Rahimov F, Attanasio C, Kouwenhoven EN, Ferreira De Lima RL, Felix TM, et al. An etiologic regulatory mutation in IRF6 with loss- and gain-of-function effects. Hum Mol Genet. 2014;23:2711–20.
Tamasas B, Cox TC. Massively increased caries susceptibility in an Irf6 cleft lip/palate model. J Dent Res. 2017;96:315–22.
Dissemond J, Haberer D, Franckson T, Hillen U. The Van der Woude syndrome: a case report and review of the literature. J Eur Acad Dermatol Venereol. 2004;18:611–3.
Oberoi S, Vargervik K. Hypoplasia and hypodontia in Van der Woude syndrome. Cleft Palate Craniofac J. 2005;42:459–66.
Tamasas B, Cox TC. Massively increased caries susceptibility in an Irf6 Cleft Lip/Palate model. J Dent Res. 2017;96:315–22.
Metwalli KA, Do MA, Nguyen K, Mallick S, Kin K, Farokhnia N, et al. Interferon regulatory factor 6 is necessary for salivary glands and pancreas development. J Dent Res. 2018;97:226–36.
Papapetrou EP, Tomishima MJ, Chambers SM, Mica Y, Reed E, Menon J, et al. Stoichiometric and temporal requirements of Oct4, Sox2, Klf4, and c-Myc expression for efficient human iPSC induction and differentiation. Proc Natl Acad Sci U S A. 2009;106:12759–64.
Khoo TS, Jamal R, Abdul Ghani NA, Alauddin H, Hussin NH, Abdul Murad NA. Retention of somatic memory associated with cell identity, age and metabolism in induced pluripotent stem (iPS) cells reprogramming. Stem Cell Rev Rep. 2020;16:251–61.
Lim JY, Yi T, Choi JS, Jang YH, Lee S, Kim HJ, et al. Intraglandular transplantation of bone marrow-derived clonal mesenchymal stem cells for amelioration of post-irradiation salivary gland damage. Oral Oncol. 2013;49:136–43.
Kojima T, Kanemaru S, Hirano S, Tateya I, Ohno S, Nakamura T, et al. Regeneration of radiation damaged salivary glands with adipose-derived stromal cells. Laryngoscope. 2011;121:1864–9.
Dodds MW, Johnson DA, Yeh CK. Health benefits of saliva: a review. J Dent. 2005;33:223–33.
Neopane P, Paudel D, Yoshida K, Adhikari BR, Morikawa T, Onishi A, et al. Immunohistochemical Localization of RNase 7 in normal and inflamed oral epithelia and salivary glands. Acta Histochem Cytochem. 2019;52:35-43.
Patel VN, Hoffman MP. Salivary gland development: a template for regeneration. Semin Cell Dev Biol. 2014;25–26:52–60.
Gao R, Cao C, Zhang M, Lopez MC, Yan Y, Chen Z, et al. A unifying gene signature for adenoid cystic cancer identifies parallel MYB-dependent and MYB-independent therapeutic targets. Oncotarget. 2014;5:12528–42.
Jaskoll T, Luo W, Snead ML. Msx-2 expression and glucocorticoid-induced overexpression in embryonic mouse submandibular glands. J Craniofac Genet Dev Biol. 1998;18:79–87.
Fowkes RC, Moradi-Bidhendi N, Brancaleone V, Zariwala MG, Brady D, Jessop DS, et al. Annexin-A1 protein and its relationship to cortisol in human saliva. Psychoneuroendocrinology. 2013;38:722–7.
Ieko T, Sasaki H, Maeda N, Fujiki J, Iwano H, Yokota H. Analysis of corticosterone and testosterone synthesis in rat salivary gland homogenates. Front Endocrinol (Lausanne). 2019;17:479.
Iwamoto N, Kawakami A, Arima K, Nakamura H, Kawashiri SY, Tamai M, et al. Regulation of disease susceptibility and mononuclear cell infiltration into the labial salivary glands of Sjögren’s syndrome by monocyte chemotactic protein-1. Rheumatology (Oxford). 2010;49:1472–8.
Dang H, Lin AL, Zhang B, Zhang HM, Katz MS, Yeh CK. Role for Notch signaling in salivary acinar cell growth and differentiation. Dev Dyn. 2009;238:724–31.
Kusafuka K, Ishiwata T, Sugisaki Y, Takemura T, Kusafuka M, Hisha H, et al. Lumican expression is associated with the formation of mesenchyme-like elements in salivary pleomorphic adenomas. J Pathol. 2004;203:953–60.
Acknowledgement
This work was supported by the National Science Foundation of China (no. 81873718).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All of the authors had no any personal, financial, commercial, or academic conflicts of interest separately.
Ethical statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the ethics committee of our Hospital (IRB no. 2020-P2-209-02).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Meng, C., Huang, S., Cheng, T. et al. Induction of Salivary Gland-Like Tissue by Induced Pluripotent Stem Cells In Vitro. Tissue Eng Regen Med 19, 389–401 (2022). https://doi.org/10.1007/s13770-021-00402-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-021-00402-8