Abstract
Background:
Engineered cell sheet transplantation has been considered an alternative physiological therapy for endocrine disorders. In this study, we attempted to fabricate functional human thyroid cell sheets using the engineering technology by culturing primary thyrocytes in free-feeder monolayers and assessed their proliferation and function in two different media.
Methods:
The non-tumorous tissues (approximately 2 g) were dissected during surgery. Primary human thyroid cells were isolated by mechanical dispersion and treatment with isolation solution. The cells were cultured on tissue culture dishes or temperature-responsive culture dishes to induce the formation of detached cell sheets.
Results:
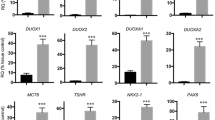
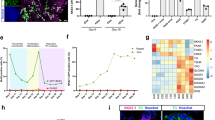
Primary thyroid cells isolated from nine patients were positive for thyroid transcription factor 1, thyroglobulin (TG) and cytokeratin 7. Cell sheets with follicles were fabricated by cells incubated in both Dulbecco’s Modified Eagle Medium (DMEM) and hepatocyte-defined medium (HDM) culture medium. The diameter and thickness of sheets fabricated in HDM were larger and thicker than those fabricated from DMEM. Furthermore, the cells incubated in HDM secreted higher levels of fT3 and fT4 than those incubated in DMEM. The thyroid peroxidase and TG mRNA of cells maintained in HDM were higher than those in cells maintained in DMEM.
Conclusion:
HDM appears suitable as a culture medium for maintaining primary thyrocytes and fabricating functional cell sheets. These in vitro findings may contribute to the development of appropriate culture conditions for human thyrocytes as well as engineered functional cell sheets.





Similar content being viewed by others
References
Celi FS, Zemskova M, Linderman JD, Smith S, Drinkard B, Sachdev V, et al. Metabolic effects of liothyronine therapy in hypothyroidism: a randomized, double-blind, crossover trial of liothyronine versus levothyroxine. J Clin Endocrinol Metab. 2011;96:3466–74.
Arauchi A, Shimizu T, Yamato M, Obara T, Okano T. Tissue-engineered thyroid cell sheet rescued hypothyroidism in rat models after receiving total thyroidectomy comparing with nontransplantation models. Tissue Eng Part A. 2009;15:3943–9.
Memon IA, Sawa Y, Fukushima N, Matsumiya G, Miyagawa S, Taketani S, et al. Repair of impaired myocardium by means of implantation of engineered autologous myoblast sheets. J Thorac Cardiovasc Surg. 2005;130:1333–41.
Ohashi K, Yokoyama T, Yamato M, Kuge H, Kanehiro H, Tsutsumi M, et al. Engineering functional two- and three-dimensional liver systems in vivo using hepatic tissue sheets. Nat Med. 2007;13:880–5.
Perrod G, Rahmi G, Pidial L, Camilleri S, Bellucci A, Casanova A, et al. Cell sheet transplantation for esophageal stricture prevention after endoscopic submucosal dissection in a porcine model. PLoS One. 2016;11:e0148249.
Sakai Y, Yamanouchi K, Ohashi K, Koike M, Utoh R, Hasegawa H, et al. Vascularized subcutaneous human liver tissue from engineered hepatocyte/fibroblast sheets in mice. Biomaterials. 2015;65:66–75.
Bell E, Moore H, Mitchie C, Sher S, Coon H. Reconstruction of a thyroid gland equivalent from cells and matrix materials. J Exp Zool. 1984;232:277–85.
Toda S, Koike N, Sugihara H. Thyrocyte integration, and thyroid folliculogenesis and tissue regeneration: perspective for thyroid tissue engineering. Pathol Int. 2001;51:403–17.
Toda S, Sugihara H. Reconstruction of thyroid follicles from isolated porcine follicle cells in three-dimensional collagen gel culture. Endocrinology. 1990;126:2027–34.
Bechtner G, Schopohl D, Rafferzeder M, Gärtner R, Welsch U. Stimulation of thyroid cell proliferation by epidermal growth factor is different from cell growth induced by thyrotropin or insulin-like growth factor I. Eur J Endocrinol. 1996;134:639–48.
Fröhlich E, Wahl R, Reutter K. Basal lamina formation by porcine thyroid cells grown in collagen- and laminin-deficient medium. Histochem J. 1995;27:602–8.
Takasu N, Ohno S, Komiya I, Yamada T. Requirements of follicle structure for thyroid hormone synthesis; cytoskeletons and iodine metabolism in polarized monolayer cells on collagen gel and in double layered, follicle-forming cells. Endocrinology. 1992;131:1143–8.
Zeng L, Geng Y, Tretiakova M, Yu X, Sicinski P, Kroll TG. Peroxisome proliferator-activated receptor-delta induces cell proliferation by a cyclin E1-dependent mechanism and is up-regulated in thyroid tumors. Cancer Res. 2008;68:6578–86.
Penna-Martinez M, Winten C, Fichtel T, Caspar-Bell G, Usadel KH, Schumm-Draeger PM. Isolation of thyroid cells obtained by fine-needle aspiration biopsy. Thyroid. 2005;15:989–95.
Baimakhanov Z, Sakai Y, Yamanouchi K, Hidaka M, Soyama A, Takatsuki M, et al. Spontaneous hepatocyte migration towards an endothelial cell tube network. J Tissue Eng Regen Med. 2018;12:e1767–71.
Toda S, Yonernitsu N, Hikichi Y, Sugihara H, Koike N. Differentiation of human thyroid follicle cells from normal subjects and Basedow’s disease in three-dimensional collagen gel culture. Pathol Res Pract. 1992;188:874–82.
Goretzki PE, Frilling A, Simon D, Roeher HD. Growth regulation of normal thyroids and thyroid tumors in man. Recent Results Cancer Res. 1990;118:48–63.
Hegyesi O, Földes A, Bori E, Németh Z, Barabás J, Steward MC, et al. Evidence for active electrolyte transport by two-dimensional monolayers of human salivary epithelial cells. Tissue Eng Part C Methods. 2015;21:1226–36.
Tiwari S, Nair RM, Vamadevan P, Ali MJ, Naik MN, Honavar SG, et al. Establishing and characterizing lacrispheres from human lacrimal gland for potential clinical application. Graefes Arch Clin Exp Ophthalmol. 2018;256:717–27.
Vassart G, Dumont JE. The thyrotropin receptor and the regulation of thyrocyte function and growth. Endocr Rev. 1992;13:596–611.
Yamazaki K, Yamada E, Kanaji Y, Shizume K, Wang DS, Maruo N, et al. Interleukin-6 (IL-6) inhibits thyroid function in the presence of soluble IL-6 receptor in cultured human thyroid follicles. Endocrinology. 1996;137:4857–63.
Colletta G, Cirafici AM, Di Carlo A. Dual effect of transforming growth factor beta on rat thyroid cells: inhibition of thyrotropin-induced proliferation and reduction of thyroid-specific differentiation markers. Cancer Res. 1989;49:3457–62.
Asmis LM, Gerber H, Kaempf J, Studer H. Epidermal growth factor stimulates cell proliferation and inhibits iodide uptake of FRTL-5 cells in vitro. J Endocrinol. 1995;145:513–20.
Westermark K, Karlsson FA, Westermark B. Epidermal growth factor modulates thyroid growth and function in culture. Endocrinology. 1983;112:1680–6.
Bravo SB, Garcia-Rendueles ME, Garcia-Rendueles AR, Rodrigues JS, Perez-Romero S, Garcia-Lavandeira M, et al. Humanized medium (h7H) allows long-term primary follicular thyroid cultures from human normal thyroid, benign neoplasm, and cancer. J Clin Endocrinol Metab. 2013;98:2431–41.
Nishida K, Yamato M, Hayashida Y, Watanabe K, Yamamoto K, Adachi E, et al. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N Engl J Med. 2004;351:1187–96.
Sawa Y, Yoshikawa Y, Toda K, Fukushima S, Yamazaki K, Ono M, et al. Safety and efficacy of autologous skeletal myoblast sheets (TCD-51073) for the treatment of severe chronic heart failure due to ischemic heart disease. Circ J. 2015;79:991–9.
Acknowledgement
This work was supported by Grant-in-Aid for Scientific Research (No. 26461950).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Ethical Statement
This study was approved by the institutional review board of the Nagasaki University Hospital (Approval Number 14052642) and we obtained informed consent by each patient.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Huang, Y., Yamanouchi, K., Sakai, Y. et al. Fabrication of Functional Cell Sheets with Human Thyrocytes from Non-Tumorous Thyroid Tissue. Tissue Eng Regen Med 16, 491–499 (2019). https://doi.org/10.1007/s13770-019-00198-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-019-00198-8