Abstract
BACKGROUND:
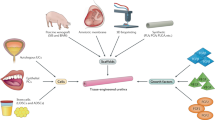
Urinary tract is subjected to a variety of disorders such as urethral stricture, which often develops as a result of scarring process. Urethral stricture can be treated by urethral dilation and urethrotomy; but in cases of long urethral strictures, substitution urethroplasty with genital skin and buccal mucosa grafts is the only option. However a number of complications such as infection as a result of hair growth in neo-urethra, and stone formation restrict the application of those grafts. Therefore, tissue engineering techniques recently emerged as an alternative approach, aiming to overcome those restrictions. The aim of this review is to provide a comprehensive coverage on the strategies employed and the translational status of urethral tissue engineering over the past years and to propose a combinatory strategy for the future of urethral tissue engineering.
METHODs:
Data collection was based on the key articles published in English language in years between 2006 and 2018 using the searching terms of urethral stricture and tissue engineering on PubMed database.
RESULTS:
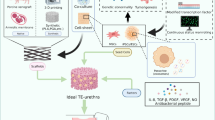
Differentiation of mesenchymal stem cells into urothelial and smooth muscle cells to be used for urologic application does not offer any advantage over autologous urothelial and smooth muscle cells. Among studied scaffolds, synthetic scaffolds with proper porosity and mechanical strength is the best option to be used for urethral tissue engineering.
CONCLUSION:
Hypoxia-preconditioned mesenchymal stem cells in combination with autologous cells seeded on a pre-vascularized synthetic and biodegradable scaffold can be said to be the best combinatory strategy in engineering of human urethra.


Similar content being viewed by others
References
Orabi H, Bouhout S, Morissette A, Rousseau A, Chabaud S, Bolduc S. Tissue engineering of urinary bladder and urethra: advances from bench to patients. ScientificWorldJournal. 2013;2013:154564.
Orabi H, Goulet CR, Fradette J, Bolduc S. Adipose-derived stem cells—are they the optimal cell source for urinary tract regeneration? In: Eberli D, editor. Cells and biomaterials in regenerative medicine. London: IntechOpen; 2014. https://doi.org/10.5772/59223.
Chung YG, Tu D, Franck D, Gil ES, Algarrahi K, Adam RM, et al. Acellular bi-layer silk fibroin scaffolds support tissue regeneration in a rabbit model of onlay urethroplasty. PLoS One. 2014;9:e91592.
de Kemp V, de Graaf P, Fledderus JO, Ruud Bosch JL, de Kort LM. Tissue engineering for human urethral reconstruction: systematic review of recent literature. PLoS One. 2015;10:e0118653.
Raya-Rivera A, Esquiliano DR, Yoo JJ, Lopez-Bayghen E, Soker S, Atala A. Tissue-engineered autologous urethras for patients who need reconstruction: an observational study. Lancet. 2011;377:1175–82.
Mangera A, Chapple CR. Tissue engineering in urethral reconstruction—an update. Asian J Androl. 2013;15:89–92.
Rogovaya OS, Fayzulin AK, Vasiliev AV, Kononov AV, Terskikh VV. Reconstruction of rabbit urethral epithelium with skin keratinocytes. Acta Naturae. 2015;7:70–7.
Oikarinen A, Sandberg M, Hurskainen T, Kinnunen T, Kallioinen M. Collagen biosynthesis in lichen sclerosus et atrophicus studied by biochemical and in situ hybridization techniques. Acta Derm Venereol Suppl (Stockh). 1991;162:3–12.
Xue JD, Gao J, Fu Q, Feng C, Xie H. Seeding cell approach for tissue-engineered urethral reconstruction in animal study: a systematic review and meta-analysis. Exp Biol Med (Maywood). 2016;241:1416–28.
Mahfouz W, Elsalmy S, Corcos J, Fayed AS. Fundamentals of bladder tissue engineering. Afr J Urol. 2013;19:51–7.
Fu Q, Deng CL, Liu W, Cao YL. Urethral replacement using epidermal cell-seeded tubular acellular bladder collagen matrix. BJU Int. 2007;99:1162–5.
Jiang S, Xu Z, Zhao Y, Yan L, Zhou Z, Gu G. Urethral reconstruction using mesothelial cell-seeded autogenous granulation tissue tube: an experimental study in male rabbits. Biomed Res Int. 2017;2017:1850256.
Bhargava S, Patterson JM, Inman RD, MacNeil S, Chapple CR. Tissue-engineered buccal mucosa urethroplasty-clinical outcomes. Eur Urol. 2008;53:1263–71.
Orabi H, Aboushwareb T, Zhang Y, Yoo JJ, Atala A. Cell-seeded tubularized scaffolds for reconstruction of long urethral defects: a preclinical study. Eur Urol. 2013;63:531–8.
Mousa NA, Abou-Taleb HA, Orabi H. Stem cell applications for pathologies of the urinary bladder. World J Stem Cells. 2015;7:815–22.
Subramaniam R, Hinley J, Stahlschmidt J, Southgate J. Tissue engineering potential of urothelial cells from diseased bladders. J Urol. 2011;186:2014–20.
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76.
Osborn SL, Thangappan R, Luria A, Lee JH, Nolta J, Kurzrock EA. Induction of human embryonic and induced pluripotent stem cells into urothelium. Stem Cells Transl Med. 2014;3:610–9.
Lv X, Guo Q, Han F, Chen C, Ling C, Chen W, et al. Electrospun poly(l-lactide)/poly(ethylene glycol) scaffolds seeded with human amniotic mesenchymal stem cells for urethral epithelium repair. Int J Mol Sci. 2016;17:E1262.
Tian H, Bharadwaj S, Liu Y, Ma PX, Atala A, Zhang Y. Differentiation of human bone marrow mesenchymal stem cells into bladder cells: potential for urological tissue engineering. Tissue Eng Part A. 2010;16:1769–79.
Liu J, Huang J, Lin T, Zhang C, Yin X. Cell-to-cell contact induces human adipose tissue-derived stromal cells to differentiate into urothelium-like cells in vitro. Biochem Biophys Res Commun. 2009;390:931–6.
Qin D, Long T, Deng J, Zhang Y. Urine-derived stem cells for potential use in bladder repair. Stem Cell Res Ther. 2014;5:69.
Liu JS, Bury MI, Fuller NJ, Sturm RM, Ahmad N, Sharma AK. Bone marrow stem/progenitor cells attenuate the inflammatory milieu following substitution urethroplasty. Sci Rep. 2016;6:35638.
Blasi A, Martino C, Balducci L, Saldarelli M, Soleti A, Navone SE, et al. Dermal fibroblasts display similar phenotypic and differentiation capacity to fat-derived mesenchymal stem cells, but differ in anti-inflammatory and angiogenic potential. Vasc Cell. 2011;3:5.
Leto Barone AA, Khalifian S, Lee WP, Brandacher G. Immunomodulatory effects of adipose-derived stem cells: fact or fiction? Biomed Res Int. 2013;2013:383685.
McIntosh K, Zvonic S, Garrett S, Mitchell JB, Floyd ZE, Hammill L, et al. The immunogenicity of human adipose-derived cells: temporal changes in vitro. Stem Cells. 2006;24:1246–53.
Li H, Xu Y, Xie H, Li C, Song L, Feng C, et al. Epithelial-differentiated adipose-derived stem cells seeded bladder acellular matrix grafts for urethral reconstruction: an animal model. Tissue Eng Part A. 2014;20:774–84.
Wen F, Chang S, Toh YC, Teoh SH, Yu H. Development of poly (lactic-co-glycolic acid)-collagen scaffolds for tissue engineering. Mater Sci Eng C Mater Biol Appl. 2007;27:285–92.
Margolis G, Polyak B, Cohen S. Magnetic induction of multiscale anisotropy in macroporous alginate scaffolds. Nano Lett. 2018;18:7314–22.
Fu WJ, Wang ZX, Li G, Zhang BH, Zhang L, Hu K, et al. A surface-modified biodegradable urethral scaffold seeded with urethral epithelial cells. Chin Med J (Engl). 2011;124:3087–92.
Stachewicz U, Szewczyk PK, Kruk A, Barber AH, Czyrska-Filemonowicz A. Pore shape dependence on cells growth into electrospun fiber scaffolds for tissue engineering: 2D and 3D analyses using SEM and FIB-SEM tomography. Mater Sci Eng C Mater Biol Appl. 2017;95:397–408.
Figallo E, Flaibani M, Zavan B, Abatangelo G, Elvassore N. Micropatterned biopolymer 3D scaffold for static and dynamic culture of human fibroblasts. Biotechnol Prog. 2007;23:210–6.
Selim M, Bullock AJ, Blackwood KA, Chapple CR, MacNeil S. Developing biodegradable scaffolds for tissue engineering of the urethra. BJU Int. 2011;107:296–302.
Lv XG, Feng C, Fu Q, Xie H, Wang Y, Huang JW, et al. Comparative study of different seeding methods based on a multilayer SIS scaffold: which is the optimal procedure for urethral tissue engineering? J Biomed Mater Res Part B Appl Biomater. 2016;104:1098–108.
Wang F, Liu T, Yang L, Zhang G, Liu H, Yi X, et al. Urethral reconstruction with tissue-engineered human amniotic scaffold in rabbit urethral injury models. Med Sci Monit. 2014;20:2430–8.
Simões IN, Vale P, Soker S, Atala A, Keller D, Noiva R, et al. Acellular urethra bioscaffold: decellularization of whole urethras for tissue engineering applications. Sci Rep. 2017;7:41934.
Feng C, Xu YM, Fu Q, Zhu WD, Cui L. Reconstruction of three dimensional neourethra using lingual keratinocytes and corporal smooth muscle cells seeded acellular corporal spongiosum. Tissue Eng Part A. 2011;17:3011–9.
Pinnagoda K, Larsson HM, Vythilingam G, Vardar E, Engelhardt EM, Thambidorai RC, et al. Acta Biomaterialia engineered acellular collagen scaffold for endogenous cell guidance, a novel approach in urethral regeneration. Acta Biomater. 2016;43:208–17.
Yan H, Zhong L, Jiang Y, Yang J, Deng J, Wei S, et al. Controlled release of insulin-like growth factor 1 enhances urethral sphincter function and histological structure in the treatment of female stress urinary incontinence in a rat model. BJU Int. 2018;121:301–12.
Raddatz L, Lavrentieva A, Pepelanova I, Bahnemann J, Geier D, Becker T, et al. Development and application of an additively manufactured calcium chloride nebulizer for alginate 3D-bioprinting purposes. J Funct Biomater. 2018;9:E63.
Wang DJ, Li MY, Huang WT, Lu MH, Hu C, Li K, et al. Repair of urethral defects with polylactid acid fibrous membrane seeded with adipose-derived stem cells in a rabbit model. Connect Tissue Res. 2015;56:434–9.
Naji M, Rasouli J, Shakhssalim N, Dehghan MM, Soleimani M. Supportive features of a new hybrid scaffold for urothelium engineering. Arch Med Sci. 2015;11:438–45.
Zhang K, Guo X, Zhao W, Niu G, Mo X, Fu Q. Application of Wnt pathway inhibitor delivering scaffold for inhibiting fibrosis in urethra strictures: in vitro and in vivo study. Int J Mol Sci. 2015;16:27659–76.
Chun SY, Kim BS, Kwon SY, Park SI, Song PH, Yoo ES, et al. Urethroplasty using autologous urethral tissue-embedded acellular porcine bladder submucosa matrix grafts for the management of long-segment urethral stricture in a rabbit model. J Korean Med Sci. 2015;30:301–7.
Sayeg K, Freitas-Filho LG, Waitzberg ÂF, Arias VE, Laks M, Egydio FM, et al. Integration of collagen matrices into the urethra when implanted as onlay graft. Int Braz J Urol. 2013;39:414–23.
Davis NF, Callanan A, McGuire BB, Flood HD, McGloughlin TM. Evaluation of viability and proliferative activity of human urothelial cells cultured onto xenogenic tissue-engineered extracellular matrices. Urology. 2011;77:1007.e1–7.
Liu Y, Ma W, Liu B, Wang Y, Chu J, Xiong G, et al. Urethral reconstruction with autologous urine-derived stem cells seeded in three-dimensional porous small intestinal submucosa in a rabbit model. Stem Cell Res Ther. 2017;8:63.
Xie M, Song L, Wang J, Fan S, Zhang Y, Xu Y. Evaluation of stretched electrospun silk fibroin matrices seeded with urothelial cells for urethra reconstruction. J Surg Res. 2013;184:774–81.
Algarrahi K, Affas S, Sack BS, Yang X, Costa K, Seager C, et al. Repair of injured urethras with silk fibroin scaffolds in a rabbit model of onlay urethroplasty. J Surg Res. 2018;229:192–9.
Tian B, Song L, Liang T, Li Z, Ye X, Fu Q, et al. Repair of urethral defects by an adipose mesenchymal stem cell—porous silk fibroin material. Mol Med Rep. 2018;18:209–15.
Sartoneva R, Haimi S, Miettinen S, Mannerström B, Haaparanta AM, Sándor GK, et al. Comparison of a poly-l-lactide-co-ε-caprolactone and human amniotic membrane for urothelium tissue engineering applications. J R Soc Interface. 2011;8:671–7.
Kaye R, Goldstein T, Grande DA, Zeltsman D, Smith LP. A 3-dimensional bioprinted tracheal segment implant pilot study: rabbit tracheal restriction with graft implantation. Int J Pediatr Otorhinolaryngol. 2019;117:175–8.
Lal H, Patralekh MK. 3D printing and its applications in orthopaedic trauma: a technological marvel. J Clin Orthop Trauma. 2018;9:260–8.
Xu T, Zhao W, Zhu JM, Albanna MZ, Yoo JJ, Atala A. Biomaterials Complex heterogeneous tissue constructs containing multiple cell types prepared by inkjet printing technology. Biomaterials. 2013;34:130–9.
Drzewiecki KE, Malavade JN, Ahmed I, Lowe CJ, Shreiber DI. A thermoreversible, photocrosslinkable collagen bio-ink for free-form fabrication of scaffolds for regenerative medicine. Technology (Singap World Sci). 2017;5:185–95.
Axpe E, Oyen ML. Applications of alginate-based bioinks in 3D bioprinting. Int J Mol Sci. 2016;17:E1976.
Bell A, Kofron M, Nistor V. Multiphoton crosslinking for biocompatible 3D printing of type I collagen. Biofabrication. 2015;7:035007.
Pati F, Jang J, Lee JW, Cho DW. Extrusion bioprinting. In: Atala A, Yoo J, editors. Essential of 3D biofabrication and translation. San Diego: Academic press Elisevier; 2015. p. 123–52.
Zhang K, Fu Q, Yoo J, Chen X, Chandra P, Mo X, et al. 3D bioprinting of urethra with PCL/PLCL blend and dual autologous cells in fibrin hydrogel: an in vitro evaluation of biomimetic mechanical property and cell growth environment. Acta Biomater. 2017;50:154–64.
Morissette A, Imbeault A, Cattan V, Bernard G, Taillon G, Chabaud S, et al. Strategies to reconstruct a functional urethral substitute by self-assembly method. Procedia Eng. 2013;59:193–200.
Magnan M, Lévesque P, Gauvin R, Dubé J, Barrieras D, El-Hakim A, et al. Tissue engineering of a genitourinary tubular tissue graft resistant to suturing and high internal pressures. Tissue Eng Part A. 2009;15:197–202.
Vallières K, Laterreur V, Tondreau MY, Ruel J, Germain L, Fradette J, et al. Human adipose-derived stromal cells for the production of completely autologous self-assembled tissue-engineered vascular substitutes. Acta Biomater. 2015;24:209–19.
Bouhout S, Gauvin R, Gibot L, Aubé D, Bolduc S. Bladder substitute reconstructed in a physiological pressure environment. J Pediatr Urol. 2011;7:276–82.
Rousseau A, Fradette J, Bernard G, Gauvin R, Laterreur V, Bolduc S. Adipose-derived stromal cells for the reconstruction of a human vesical equivalent. J Tissue Eng Regen Med. 2013;9:E135–43.
Seifarth V, Grosse JO, Gossmann M, Janke HP, Arndt P, Koch S, et al. Mechanical induction of bi-directional orientation of primary porcine bladder smooth muscle cells in tubular fibrin-poly(vinylidene fluoride) scaffolds for ureteral and urethral repair using cyclic and focal balloon catheter stimulation. J Biomater Appl. 2017;32:321–30.
Cattan V, Bernard G, Rousseau A, Bouhout S, Chabaud S, Auger FA, et al. Mechanical stimuli-induced urothelial differentiation in a human tissue-engineered tubular genitourinary graft. Eur Urol. 2011;60:1291–8.
Vardar E, Engelhardt EM, Larsson HM, Mouloungui E, Pinnagoda K, Hubbell JA, et al. Tubular compressed collagen scaffolds for ureteral tissue engineering in a flow bioreactor system. Tissue Eng Part A. 2015;21:2334–45.
Imbeault A, Bernard G, Rousseau A, Morissette A, Chabaud S, Bouhout S, et al. An endothelialized urothelial cell-seeded tubular graft for urethral replacement. Can Urol Assoc J. 2013;7:E4–9.
Zhou S, Yang R, Zou Q, Zhang K, Yin T, Zhao W, et al. Fabrication of tissue-engineered bionic urethra using cell sheet technology and labeling by ultrasmall superparamagnetic iron oxide for full-thickness urethral reconstruction. Theranostics. 2017;7:2509–23.
Sun D, Yang Y, Wei Z, Xu Y, Zhang X, Hong B. Engineering of pre-vascularized urethral patch with muscle flaps and hypoxia-activated hUCMSCs improves its therapeutic outcome. J Cell Mol Med. 2014;18:434–43.
Liu J, Hao H, Huang H, Tong C, Ti D, Dong L, et al. Hypoxia regulates the therapeutic potential of mesenchymal stem cells through enhanced autophagy. Int J Low Extrem Wounds. 2015;14:63–72.
Li C, Xu YM, Liu ZS, Li HB. Urethral reconstruction with tissue engineering and RNA interference techniques in rabbits. Urology. 2013;81:1075–80.
Chen KL, Wu HC, Chang CH. Tissue-engineered constructs for urethral regeneration. Urol Sci. 2012;23:42–4.
El-Tabey N, Shokeir A, Barakat N, El-Refaie H, El-Hamid MA, Gabr M. Cell-seeded tubular acellular matrix for replacing a long circumferential urethral defect in a canine model: is it clinically applicable? Arab J Urol. 2012;10:192–8.
Fu Q, Deng CL, Song XF, Xu YM. Long-term study of male rabbit urethral mucosa reconstruction using epidermal cell. Asian J Androl. 2008;10:719–22.
Osman NI, Patterson JM, MacNeil S, Chapple CR. Long-term follow-up after tissue-engineered buccal mucosa urethroplasty. Eur Urol. 2014;66:790–1.
da Silva EA, Sampaio FJ, Ortiz V, Cardoso LE. Regional differences in the extracellular matrix of the human spongy urethra as evidenced by the composition of glycosaminoglycans. J Urol. 2002;167:2183–7.
Acknowledgement
This review paper was supported by grants from Universiti Kebangsaan Malaysia (GUP-2017-092 and FF-2017-227).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no financial conflicts of interest regarding the publication of this paper.
Ethical statement
There are no animal and human experiments carried out for this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rashidbenam, Z., Jasman, M.H., Hafez, P. et al. Overview of Urethral Reconstruction by Tissue Engineering: Current Strategies, Clinical Status and Future Direction. Tissue Eng Regen Med 16, 365–384 (2019). https://doi.org/10.1007/s13770-019-00193-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-019-00193-z