Abstract
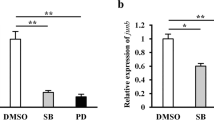
Epimorphic regeneration in vertebrates involves the restoration of lost tissue or organs through the formation of a regeneration blastema and occurs through a complex interaction of a number of molecular signaling pathways. Of the many effectors of successful tail regeneration in the lizard Hemidactylus flaviviridis, one crucial pathway is the cyclooxygenase-2 (COX-2) mediated PGE2 signaling pathway. The current study was aimed at understanding whether COX-2 signaling plays any role in the expression of Wnt/β-Catenin signaling components during regenerative outgrowth in H. flaviviridis. Etoricoxib—selective inhibitor of the inducible isoform of COX-2—was administered to lizards orally. We tested the expression of β-Catenin during wound epidermis and blastema stages in the regenerating tail and found a reduction in its expression in response to drug treatment. Further, it was observed that the expression of canonical Wnt ligands was greatly altered due to COX-2 inhibition. Our results provide evidence of a cross-talk between the COX-2 induced PGE2 pathway and Wnt/β-Catenin signaling in the regenerating lizard tail. An understanding of the interaction among various signaling pathways will help elucidate the mechanism underlying epimorphosis in lizards, the only amniotes capable of appendage regeneration.






Similar content being viewed by others
References
Wallace H. Vertebrate limb regeneration. Chichester: Wiley; 1981.
Call MK, Tsonis PA. Vertebrate limb regeneration. Adv Biochem Eng Biotechnol. 2005;93:67–81.
Stoick-Cooper C, Weidinger G, Riehle KJ, Hubbert C, Major MB, Fausto N, Moon RT. Distinct Wnt signaling pathways have opposing roles in appendage regeneration. Development. 2007;134:479–89.
Kumar A, Pilo B. Influence of catecholamine and acetylcholine neurotransmitters on tail regeneration in gekkonid lizard Hemidactylus flaviviridis. Ind J Exp Biol. 1994;32:767–71.
Pilo B, Suresh B. Effect of EGF on progress of tail regeneration in the gekkonid lizard Hemidactylus flaviviridis. J Anim Morph Physiol. 1994;41:61–6.
Pilo B, Kumar A. Effect of chemical adrenalectomy and corticosterone administration on tail regeneration in the gekkonid lizard Hemidactylus flaviviridis. Ind J Exp Biol. 1995;33:917–20.
Sharma P, Suresh B. Influence of COX-2-induced PGE on the initiation and progression of tail regeneration in Northern House Gecko, Hemidactylus Flaviviridis. Folia Biol (Praha). 2008;54:193–200.
Yadav M, Buch P, Desai I, Suresh B. Exogenous administration of EGF augments nucleic acid biosynthesis and cell proliferation in the regenerating tail of wall lizard. Eur J Exp Biol. 2014;4:113–23.
Smith WL. Prostanoid biosynthesis and mechanisms of action. Am J Physiol. 1992;263:F181–91.
Sugimoto Y, Narumiya S. Prostaglandin E receptors. J Biol Chem. 2007;282:11613–7.
Greenhough A, Smartt HJM, Moore AE, Roberts HR, Williams AC, Paraskeva C, Kaidi A. The COX-2/PGE 2 pathway: key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis. 2009;30:377–86.
Saini M, Sanyal S. Evaluation of chemopreventive response of two Cycloxygenase-2 inhibitors, Etoricoxib and Diclofenac in rat colon cancer using FTIR and NMR spectroscopic techniques. Nutr Hosp. 2010;25:577–85.
Li W, Yue W, Zhang L, Zhao X, Ma L, Yang X, Zhang C, Wang Y, Gu M. COX-2 silencing inhibits proliferation in A549 cell. Chin Ger J Clin Oncol. 2011;10:P423–7.
Finetti F, Solito R, Morbidelli L, Giachetti A, Ziche M, Donnini S. Prostaglandin E2 regulates angiogenesis via activation of fibroblast growth factor receptor-1. J Biol Chem. 2008;283:2139–46.
Veliça P, Bunce CM. Prostaglandins in muscle regeneration. J Muscle Res Cell Motil. 2008;29:163–7.
Anusree P, Saradamba A, Tailor N, Desai I, Suresh B. Caudal fin regeneration is regulated by Cox-2 induced PGE2 in Teleost fish Poecilia latipinna. J Cell Tissue Res. 2011;11:2795–801.
Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509.
Brautigam L, Nefflen JU, Geisslinger G. Determination of Etoricoxib in human plasma by liquid chromatography–tandem mass spectrometry with electrospray ionisation. J Chromatogr B. 2003;788:309–15.
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods. 2001;25:402–8.
Wong CT, Ahmad E, Li H, Crawford DA. Prostaglandin E2 alters Wnt-dependent migration and proliferation in neuroectodermal stem cells: implications for autism spectrum disorders. Cell Commun Signal. 2014;12:19.
Godwin J, Kuraitis D, Rosenthal N. Extracellular matrix considerations for scar-free repair and regeneration: insights from regenerative diversity among vertebrates. Int J Biochem Cell Biol. 2014;56:47–55.
Suresh B, Sharma P, Desai I. Evidence of the involvement of Prostaglandin E2 in different cellular activities during epimorphic regeneration. J Cell Tissue Res. 2009;9:1883–90.
Labus MB, Stirk CM, Thompson WD, Melvin WT. Expression of Wnt genes in early wound healing. Wound Repair Regen. 1998;6:58–64.
Otto A, Schmidt C, Luke G, Allen S, Valasek P, Muntoni F, Lawrence-Watt D, Patel K. Canonical Wnt signalling induces satellite-cell proliferation during adult skeletal muscle regeneration. J Cell Sci. 2008;121:2939–50. doi:10.1242/jcs.026534.
Caubit X, Nicolas S, Le Parco Y. Possible roles for Wnt genes in growth and axial patterning during regeneration of the tail in urodele amphibians. Dev Dyn. 1997;210:1–10.
Caubit X, Nicolas S, Shi D, Le Parco Y. Reactivation and graded axial expression pattern of Wnt-10a gene during early regeneration stages of adult tail in amphibian urodele Pleurodeles waltl. Dev Dyn. 1997;208:139–48.
Yokoyama H, Ogino H, Stoick-Cooper CL, Grainger RM, Moon RT. Wnt/β-catenin signaling has an essential role in the initiation of limb regeneration. Dev Biol. 2007;306:170–8.
Shao J, Jung C, Liu C, Sheng H. Prostaglandin E2 stimulates the β-catenin/T cell factor-dependent transcription in colon cancer. J Biol Chem. 2005;280:26565–72.
North TE, Babu IR, Veddera LM, Lord AM, Wishnok JS, Tannenbaum SR, Zon LI, Goessling W. PGE2-regulated Wnt signaling and N-acetylcysteine are synergistically hepatoprotective in zebrafish acetaminophen injury. Proc Natl Acad Sci. 2010;107:17315–20.
Liu X, Kirschenbaum A, Weinstein BM, Zaidi M, Yao S, Levine AC. Prostaglandin E2 modulates components of the Wnt signaling system in bone and prostate cancer cells. Biochem Biophys Res Commun. 2010;394:715–20.
MacDonald BT, Tamai K, He X. Wnt/β-catenin signailng: components. Mech Dis Dev Biol. 2010;17:9–26.
Ramos-Solano M, Meza-Canales ID, Torres-Reyes LA, Alvarez-Zavala M, Alvarado-Ruíza L, Rincon-Orozcod B, Garcia-Chagollan M, Ochoa-Hernández AB, Ortiz-Lazareno PC, Rösld F, Gariglio P, Jave-Suárez LF, Aguilar-Lemarroy A. Expression of WNT genes in cervical cancer-derived cells: implication of WNT7A in cell proliferation and migration. Exp Cell Res. 2015;335:39–50.
Lyu J, Joo C. Wnt-7a up-regulates matrix metalloproteinase-12 expression and promotes cell proliferation in corneal epithelial cells during wound healing. J Biol Chem. 2005;280:21653–60.
Qu Q, Sun G, Li W, Yang S, Ye P, Zhao C, Yu RT, Gage FH, Evans RM, Shi Y. Orphan nuclear receptor TLX activates Wnt/β-catenin signalling to stimulate neural stem cell proliferation and self-renewal. Nat Cell Biol. 2010;12:1–20.
Stulberg MJ, Lin A, Zhao H, Holley SA. Crosstalk between Fgf and Wnt signaling in the zebrafish tailbud. Dev Biol. 2012;369:298–307.
Acknowledgements
The authors are thankful to DST-SERB, Govt. of India for financial assistance in the form of Project No. SB/SO/AS-008; CSIR, New Delhi for research fellowship to PB; DBT-MSUB-ILSPARE, The M. S. University of Baroda for providing the LC/MS facility; Adityarao Khnavilkar for his assistance in LC/MS.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest whatsoever.
Ethical statement
All experimental protocols were approved by the Institutional Animal Ethics Committee (IAEC) constituted as per the guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), India (IACUC no. 984/07/2014-2).
Rights and permissions
About this article
Cite this article
Buch, P.R., Sarkate, P., Uggini, G.K. et al. Inhibition of Cyclooxygenase-2 Alters Wnt/β-Catenin Signaling in the Regenerating Tail of Lizard Hemidactylus flaviviridis . Tissue Eng Regen Med 14, 171–178 (2017). https://doi.org/10.1007/s13770-017-0037-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-017-0037-2