Abstract
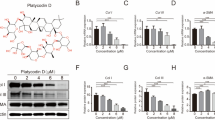
To evaluate whether Palmitoyl-pentapeptide (Pal-KTTKS), a lipidated subfragment of type 1 pro-collagen (residues 212–216), plays a role in fibroblast contractility, the effect of Pal-KTTKS on the expression of pro-fibrotic mediators in hypertropic scarring were investigated in relation with trans-differentiation of fibroblast to myofibroblast, an icon of scar formation. α-SMA was visualized by immunofluorescence confocal microscopy with a Cy-3-conjugated monoclonal antibody. The extent of α-SMA-positive fibroblasts was determined in collagen lattices and in cell culture study. Pal-KTTKS (0–0.5 µM) induced CTGF and α-SMA protein levels were determined by western blot analysis and fibroblast contractility was assessed in three-dimensional collagen lattice contraction assay. In confocal analysis, fibroblasts were observed as elongated and spindle shapes while myofibroblast observed as squamous, enlarged cells with pronounced stress fibers. Without Pal-KTTKS treatment, three quarters of the fibroblasts differentiates into the myofibroblast; α-SMA-positive stress fibers per field decreased twofold with 0.1 µM Pal-KTTKS treatment (75 ± 7.1 vs 38.6 ± 16.1%, n = 3, p < 0.05). The inhibitory effect was not significant in 0.5 µM Pal-KTTKS treatment. Stress fiber level and collagen contractility correlates with α-SMA expression level. In conclusion, Pal-KTTKS (0.1 µM) reduces α-SMA expression and trans-differentiation of fibroblasts to myofibroblast. The degree of reduction is dose-dependent. An abundance of myofibroblast and fibrotic scarring is correlated with excessive levels of α-SMA and collagen contractility. Delicate balance between the wound healing properties and pro-fibrotic abilities of pentapeptide KTTKS should be considered for selecting therapeutic dose for scar prevention.





Similar content being viewed by others
References
Katayama K, Armendariz Borunda J, Raghow R. A pentapeptide from type 1 procollagen promotes extracellular matrix production. J Biol Chem. 1993;268:9941–4.
Castelletto V, Hamley IW, Perez J, Abezgauz L, Danino D. Fibrillar superstructure from extended nanotapes formed by a collagen-stimulating peptide. Chem Commun. 2010;46:9185–87.
Tsai WC, Hsu CC, Chung CY, Lin MS, Li MS, Pang JH. The pentapeptide KTTKS promoting the expressions of type 1 collagen and transforming growth factor-β of tendon cells. J Orthop Res. 2007;25:1629–34.
Jones RR, Castelletto V, Connon CJ, Hamley IW. Collagen stimulating effect of peptide amphiphile C-16-KTTKS on human fibroblasts. Mol Pharm. 2013;10:1063–9.
Juan FM, Beatriu E, Maria DS-M, Marta TS, Ian WH, Ashkan D, et al. Self-assembly of a peptide amphiphile: transition from nanotape fibrils to micelles. Soft Matter. 2013;9:3558–64.
Farwick M, Grether-Beck S, Marini A, Maczkiewitz U, Lange J, Kohler T, et al. Bioactive tetrapeptide GEKG boosts extracellular matrix formation: in vitro and in vivo molecular and clinical proof. Exp Dermatol. 2011;20:600–13.
Maquart FX, Pasco S, Ramont L, Hornebeck W, Monboisse JC. An introduction to matrikines: extracellular matrix-derived peptides which regulate cell activity implication in tumor invasion. Crit Rev Oncol Hematol. 2004;49:199–202.
Roanne RJ, Valeria C, Che JC, Ian WH. Collagen stimulating effect of peptide amphiphile C 16-KTTKS on human fibroblasts. Mol Pharm. 2013;10:1063–9.
Abu Samah NH, Heard CM. Topically applied KTTKS: a review. Int J Cosmet Sci. 2011;33:483–90.
Reid R, Mogford JE, Butt R, Miller AG, Mustoe T. Inhibition of procollagen C-proteinase reduces scar hyperthrophy in a rabbit model of cutaneous scarring. Wound Rep Regen. 2006;14:138–41.
Yang M, Huang H, Li J, Huang W, Wang H. Connective tissue growth factor increases matrix metalloproteinase-2 and suppresses tissue inhibitor of matrix metalloproteinase-2 production by cultured renal interstitial fibroblasts. Wound Rep Regen. 2007;15:817–24.
Alfaro MP, Deskins DL, Wallus M, DasGupta J, Davidson JM, Nanney LB, et al. A physiological role for connective tissue growth factor in early wound healing. Lab Invest. 2013;93:81–95.
Frazier K, Williams S, Kothapalli D, Klapper H, Grotendors GR. Stimulation of fibroblast cell growth, matrix production and granulation tissue formation by CTGF. J Invest Dermatol. 1996;107:404–11.
Leask A, Holmes A, Abraham DJ. Connective tissue growth factor: a new and important player in the pathogenesis of fibrosis. Curr Rheumatol Rep. 2002;4:136–42.
Sonnylal S, Shi-wen X, Leoni P, Naff K, Van Pelt C, Nakamura H, et al. Selective expression of CTGF in fibroblasts in vivo promotes systemic tissue fibrosis. Arthritis Rheum. 2010;62:1523–32.
Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol. 2007;127:526–37.
Hinz B, Celetta G, Tomasek JJ, Gabbiani G, Chaponnier C. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol Biol Cell. 2001;12:2730–41.
Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol. 2003;200:500–3.
Daniels JT, Schultz GS, Blalock TD, Garrett Q, Grotendorst GR, Dean NM, et al. Mediation of transforming growth factor-β1-stimulated matrix contraction by fibroblasts: a role for CTGF in contractile scarring. Am J Pathol. 2003;163:2043–52.
Desai VD, Hsia HC, Schwarzbauer JE. Reversible modulation of myofibroblast differentiation in adipose-derived mesenchymal stem cells. PLoS ONE. 2014;9:e86865.
Evans RA, Tian YC, Steadman R, Phillips AO. TGF-1-mediated fibroblast-myofibroblast terminal differentiation—the role of Smad proteins. Exp Cell Res. 2003;282:90–100.
Moon HK, Yong HY, Cho Lee AR. Optimum scratch assay condition to evaluate CTGF expression for anti-scar therapy. Arch Pharm Res. 2012;35:383–8.
Tang J, Sato T. Effect of type I collagen derivative from Tilapia Scale on odontobalst-like cells. Tissue Eng Regen Med. 2015;12:231–8.
Diegelman RF, Evans MC. Wound Healing: an overview of acute, Fibrotic and delayed healing. Front Biosci. 2004;9:283–9.
Boris H, Giuseppe C, James JT, Giulio G, Christine C. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol Biol Cell. 2001;12:2730–41.
Rinkevich Y, Walmsley G, Hu M, Maan A, Newman A, Drukker M, et al. Identification and isolation of a dermal lineage with intrinsic fibrogenic potential. Science 2015;348(6232). doi:10.1126/science.aaa2151.
Mas-Chamberlin C, Mondon P, Peschard O, Lintner K. Matrikine technology and barrier repair: the ultimate in anti-age skin care? Cosmetic Sci Technol 2004;53.
Dehsorkhi A, Castelletto V, Hamley IW. Self-assembling amphiphilic peptides. J Pept Sci. 2014;20:453–67.
Maquart FX, Bellon G, Pasco S, Monboisse JC. Matrikines in the regulation of extracellular matrix degradation. Biochimie. 2005;87:353–60.
Choi YL, Park EJ, Kim E, Na DH, Shin YH. Dermal stability and in vitro skin permeation of collagen pentapeptides (KTTKS). Biomol Ther. 2014;22:321–7.
Acknowledgements
This work was supported by the Duksung Women’s University Research Grants 2014. We thank Prof. Kyung Chan Park at Department of Dermatology, School of Medicine, Seoul National University for his valuable discussion and for providing primary human dermal fibroblasts.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest statement
The authors declare no conflict of interest with any person or any organization.
Ethical statement
There are no animal experiments carried out for this study.
Rights and permissions
About this article
Cite this article
Park, H., An, E. & Cho Lee, AR. Effect of Palmitoyl-Pentapeptide (Pal-KTTKS) on Wound Contractile Process in Relation with Connective Tissue Growth Factor and α-Smooth Muscle Actin Expression. Tissue Eng Regen Med 14, 73–80 (2017). https://doi.org/10.1007/s13770-016-0017-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-016-0017-y