Abstract
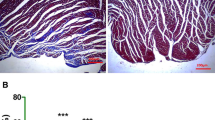
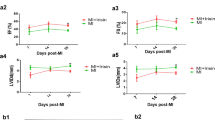
In ischemic heart disease, cardiomyocyte death results from both necrosis and apoptosis, and the area of infarction gradually increases. The induction of therapeutic angiogenesis is a promising therapy for this condition. In the present study, we investigated the actions of SV peptide—a 7-amino-acid sequence with angiogenic properties derived from osteopontin in the extracellular matrix—in a rat model of myocardial ischemia. We examined the angiogenesis activity of SV ex vivo using ring assay and dorsal air sac assay and in ischemic myocardium. We also conducted histological evaluations of left-ventricle remodeling and evaluated left ventricular (LV) function by echocardiography. The SV peptide had strong angiogenic effects both ex vivo and in vivo. In the experimental rats, this peptide stimulated the formation of new blood vessels in ischemic myocardium. Histological evaluation of the left ventricle in the SV-treated group showed a significant decrease in the size of the infarction, rate of myocardial fibrosis, and cardiomyocyte hypertrophy. Moreover, administration of the SV peptide significantly improved LV function. These results indicate that SV peptide induced angiogenesis in ischemic myocardium and improved cardiac function. Thus, it could serve as a potentially useful new therapy for ischemic heart disease.
Similar content being viewed by others
References
Aoki M, Morishita R, Taniyama Y, Kida I, Moriguchi A, Matsumoto K, et al. Angiogenesis induced by hepatocyte growth factor in non-infarcted myocardium and infarcted myocardium: up-regulation of essential transcription factor for angiogenesis, ets. Gene Ther 2000;7:417–427.
Tomita S, Li RK, Weisel RD, Mickle DA, Kim EJ, Sakai T, et al. Autologous transplantation of bone marrow cells improves damaged heart function. Circulation 1999;100(19 Suppl):II247–II256.
Yang J, Yamato M, Kohno C, Nishimoto A, Sekine H, Fukai F, et al. Cell sheet engineering: recreating tissues without biodegradable scaffolds. Biomaterials 2005;26:6415–6422.
Yoo KJ, Li RK, Weisel RD, Mickle DA, Li G, Yau TM. Autologous smooth muscle cell transplantation improved heart function in dilated cardiomyopathy. Ann Thorac Surg 2000;70:859–865.
Jayasankar V, Woo YJ, Bish LT, Pirolli TJ, Chatterjee S, Berry MF, et al. Gene transfer of hepatocyte growth factor attenuates postinfarction heart failure. Circulation 2003;108 Suppl 1:II230–II236.
Kunugiza Y, Tomita N, Taniyama Y, Tomita T, Osako MK, Tamai K, et al. Acceleration of wound healing by combined gene transfer of hepatocyte growth factor and prostacyclin synthase with Shima Jet. Gene Ther 2006; 13:1143–1152.
Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1995;1:27–31.
Haider HKh, Jiang S, Idris NM, Ashraf M. IGF-1-overexpressing mesenchymal stem cells accelerate bone marrow stem cell mobilization via paracrine activation of SDF-1alpha/CXCR4 signaling to promote myocardial repair. Circ Res 2008;103:1300–1308.
Iwanaga K, Takano H, Ohtsuka M, Hasegawa H, Zou Y, Qin Y, et al. Effects of G-CSF on cardiac remodeling after acute myocardial infarction in swine. Biochem Biophys Res Commun 2004;325:1353–1359.
Takano H, Qin Y, Hasegawa H, Ueda K, Niitsuma Y, Ohtsuka M, et al. Effects of G-CSF on left ventricular remodeling and heart failure after acute myocardial infarction. J Mol Med (Berl) 2006;84:185–193.
Ghostine S, Carrion C, Souza LC, Richard P, Bruneval P, Vilquin JT, et al. Long-term efficacy of myoblast transplantation on regional structure and function after myocardial infarction. Circulation 2002;106(12 Suppl 1): I131–I136.
Chen XH, Minatoguchi S, Kosai K, Yuge K, Takahashi T, Arai M, et al. In vivo hepatocyte growth factor gene transfer reduces myocardial ischemia- reperfusion injury through its multiple actions. J Card Fail 2007;13: 874–883.
Li Y, Takemura G, Kosai K, Yuge K, Nagano S, Esaki M, et al. Postinfarction treatment with an adenoviral vector expressing hepatocyte growth factor relieves chronic left ventricular remodeling and dysfunction in mice. Circulation 2003;107:2499–2506.
Wai PY, Kuo PC. The role of Osteopontin in tumor metastasis. J Surg Res 2004;121:228–241.
Ruvinov E, Leor J, Cohen S. The promotion of myocardial repair by the sequential delivery of IGF-1 and HGF from an injectable alginate biomaterial in a model of acute myocardial infarction. Biomaterials 2011;32: 565–578.
Taniyama Y, Morishita R, Aoki M, Hiraoka K, Yamasaki K, Hashiya N, et al. Angiogenesis and antifibrotic action by hepatocyte growth factor in cardiomyopathy. Pertension 2002;40:47–53.
Krishnamurthy P, Peterson JT, Subramanian V, Singh M, Singh K. Inhibition of matrix metalloproteinases improves left ventricular function in mice lacking osteopontin after myocardial infarction. Mol Cell Biochem 2009;322:53–62.
Kim JH, Jung Y, Kim SH, Sun K, Choi J, Kim HC, et al. The enhancement of mature vessel formation and cardiac function in infarcted hearts using dual growth factor delivery with self-assembling peptides. Biomaterials 2011;32:6080–6088.
Hamada Y, Nokihara K, Okazaki M, Fujitani W, Matsumoto T, Matsuo M, et al. Angiogenic activity of osteopontin-derived peptide SVVYGLR. Biochem Biophys Res Commun 2003;310:153–157.
Denhardt DT, Noda M, O’Regan AW, Pavlin D, Berman JS. Osteopontin as a means to cope with environmental insults: regulation of inflammation, tissue remodeling, and cell survival. J Clin Invest 2001;107:1055- 1061.
Denhardt DT, Noda M. Osteopontin expression and function: role in bone remodeling. J Cell Biochem Suppl 1998;30–31:92–102.
Miyagawa S, Sawa Y, Fukuda K, Hisaka Y, Taketani S, Memon IA, et al. Angiogenic gene cell therapy using suicide gene system regulates the effect of angiogenesis in infarcted rat heart. Transplantation 2006;81:902–907.
Trueblood NA, Xie Z, Communal C, Sam F, Ngoy S, Liaw L, et al. Exaggerated left ventricular dilation and reduced collagen deposition after myocardial infarction in mice lacking osteopontin. Circ Res 2001;88:1080–1087.
Miyagi Y, Chiu LL, Cimini M, Weisel RD, Radisic M, Li RK. Biodegradable collagen patch with covalently immobilized VEGF for myocardial repair. Biomaterials 2011;32:1280–1290.
Yokosaki Y, Matsuura N, Sasaki T, Murakami I, Schneider H, Higashiyama S, et al. The integrin alpha(9)beta(1) binds to a novel recognition sequence (SVVYGLR) in the thrombin-cleaved amino-terminal fragment of osteopontin. J Biol Chem 1999;274:36328–36334.
Yokosaki Y, Palmer EL, Prieto AL, Crossin KL, Bourdon MA, Pytela R, et al. The integrin alpha 9 beta 1 mediates cell attachment to a non-RGD site in the third fibronectin type III repeat of tenascin. J Biol Chem 1994;269:26691–26696.
Yokosaki Y, Tanaka K, Higashikawa F, Yamashita K, Eboshida A. Distinct structural requirements for binding of the integrins alphavbeta6, alphavbeta3, alphavbeta5, alpha5beta1 and alpha9beta1 to osteopontin. Matrix Biol 2005;24:418–427.
Hamada Y, Yuki K, Okazaki M, Fujitani W, Matsumoto T, Hashida MK, et al. Osteopontin-derived peptide SVVYGLR induces angiogenesis in vivo. Dent Mater J 2004;23:650–655.
Mori S, Tran V, Nishikawa K, Kaneda T, Hamada Y, Kawaguchi N, et al. A dominant-negative FGF1 mutant (the R50E mutant) suppresses tumorigenesis and angiogenesis. PLoS One 2013;8:e57927.
Beauvais DM, Ell BJ, McWhorter AR, Rapraeger AC. Syndecan-1 regulates alphavbeta3 and alphavbeta5 integrin activation during angiogenesis and is blocked by synstatin, a novel peptide inhibitor. J Exp Med 2009; 206:691–705.
Stupack DG, Cheresh DA. Get a ligand, get a life: integrins, signaling and cell survival. J Cell Sci 2002;115(Pt 19):3729–3738.
Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature 2000;407:249–257.
Uchinaka A, Kawaguchi N, Hamada Y, Mori S, Miyagawa S, Saito A, et al. Transplantation of myoblast sheets that secrete the novel peptide SVVYGLR improves cardiac function in failing hearts. Cardiovasc Res 2013; 99:102–110.
Ross RS. The extracellular connections: the role of integrins in myocardial remodeling. J Card Fail 2002;8(6 Suppl):S326–S331.
Mehrabi MR, Serbecic N, Tamaddon F, Huber K, Pacher R, Grimm M, et al. Revascularization of myocardial scar tissue following prostaglandin E1-therapy in patients with ischemic heart disease. Pathol Res Pract 2003; 199:129–136.
Ueda H, Nakamura T, Matsumoto K, Sawa Y, Matsuda H, Nakamura T. A potential cardioprotective role of hepatocyte growth factor in myocardial infarction in rats. Cardiovasc Res 2001;51:41–50.
Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell 2002;110:673–687.
Yokosaki Y, Matsuura N, Higashiyama S, Murakami I, Obara M, Yamakido M, et al. Identification of the ligand binding site for the integrin alpha9 beta1 in the third fibronectin type III repeat of tenascin-C. J Biol Chem 1998;273:11423–11428.
Ross RS, Borg TK. Integrins and the myocardium. Circ Res 2001;88:1112–1119.
Author information
Authors and Affiliations
Corresponding author
Additional information
The first 2 authors contributed equally to this work.
Rights and permissions
About this article
Cite this article
Uchinaka, A., Hamada, Y., Mori, S. et al. Cardioprotective effects on ischemic myocardium induced by SVVYGLR peptide via its angiogenic-promoting activity. Tissue Eng Regen Med 12, 162–171 (2015). https://doi.org/10.1007/s13770-015-0087-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-015-0087-2