Abstract
Purpose
The aim of this study is to evaluate proliferation, migration, and various gene expressions including insulin-like growth factor 1 (IGF-1), SMAD3, and collagen type I (COL1A1) during light-emitting diode (LED) irradiation of human gingival fibroblasts (HGF).
Materials and methods
HGF was seeded on the culture dish and stimulated using white (1.26 J/cm2) and red (0.3 J/cm2) wavelength LED for 9 min during 3 days. The distance between LED light and HGF was about 30 mm. To evaluate cell proliferation, MTT assay was carried out for 3 days after LED irradiation. After culturing for 1 and 3 days, cells were harvested and total RNA was isolated. To assess the expression of IGF-1, IGF-2, IGF-1R, SMAD3, COL1A1, and GAPDH, cDNA was synthesized and RT-PCR was performed. Whether mixed LED irradiation affect to intracellular adhesion molecule-1 (ICAM-1) mRNA expression of HGF, real time PCR was performed. To measure wound healing after LED irradiation, straight scratch was made and observed migrated cells to the scratched region using microscope. Transwell migration assay was carried out to measure migrated cells after LED irradiation. Migrated cells were stained using hematoxilin.
Results
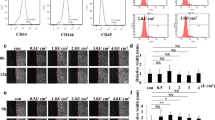
Cell proliferation was significantly increased both 1 and 3 days after LED irradiation. IGF-1 and COL1A1 mRNA expression was increased in LED irradiated cells compared to control cells. However, IGF-1R, IGF-2, and SMAD3 expression was decreased in LED irradiated cells compare to control cells. In LED irradiated cells, ICAM-1 expression was decreased than control. Interestingly, LED irradiation dramatically promoted wound healing and cell migration compared to control cells. Especially after 20 h, most of the substrate was covered by migrated HGF after LED irradiation.
Conclusion
These results suggest that simultaneous use of red and white wavelength LED promotes proliferation and migration of human gingival fibroblasts in vitro.
Similar content being viewed by others
References
Bartold, P.M. and A.S. Narayanan, Molecular and cell biology of healthy and diseased periodontal tissues. Periodontol 2000, 40, 29 (2006).
Preshaw, P.M. and J.J. Taylor, How has research into cytokine interactions and their role in driving immune responses impacted our understanding of periodontitis? Journal of Clinical Periodontology, 38, 60 (2011).
Barrientos, S., et al., Growth factors and cytokines in wound healing. Wound Repair Regen, 16, 585 (2008).
Chang, P.C., et al., Irradiation by light-emitting diode light as an adjunct to facilitate healing of experimental periodontitis in vivo. J Periodontal Res, 48, 135 (2013).
Cobb, C.M., Lasers in periodontics: a review of the literature. J Periodontol, 77, 545 (2006).
Conlan, M.J., J.W. Rapley, and C.M. Cobb, Biostimulation of wound healing by low-energy laser irradiation. A review. J Clin Periodontol, 23, 492 (1996).
Weiss, R.A., et al., Clinical trial of a novel non-thermal LED array for reversal of photoaging: Clinical, histologic and surface profilometric results. Lasers in Surgery and Medicine, 36, 85 (2005).
Chang, P.C., C.Y. Wang, and L.Y. Chong, Controlling periodontal bone levels with multiple LED irradiations. Lasers Med Sci, (2013).
de Paula Eduardo, C., et al., Laser phototherapy in the treatment of periodontal disease. A review. Lasers Med Sci, 25, 781 (2010).
AlGhamdi, K.M., A. Kumar, and N.A. Moussa, Low-level laser therapy: a useful technique for enhancing the proliferation of various cultured cells. Lasers Med Sci, 27, 237 (2012).
Oliveira Sampaio, S.C., et al., Effect of laser and LED phototherapies on the healing of cutaneous wound on healthy and iron-deficient Wistar rats and their impact on fibroblastic activity during wound healing. Lasers Med Sci, 28, 799 (2013).
Barolet, D., Light-emitting diodes (LEDs) in dermatology. Semin Cutan Med Surg, 27, 227 (2008).
Amorim, J.C., et al., Clinical study of the gingiva healing after gingivectomy and low-level laser therapy. Photomed Laser Surg, 24, 588 (2006).
Spitler, R. and M.W. Berns, Comparison of laser and diode sources for acceleration of in vitro wound healing by low-level light therapy. J Biomed Opt, 19, 38001 (2014).
Ayuk, S.M., N.N. Houreld, and H. Abrahamse, Collagen production in diabetic wounded fibroblasts in response to low-intensity laser irradiation at 660 nm. Diabetes Technol Ther, 14,1110 (2012).
Almeida-Lopes, L., et al., Comparison of the low level laser therapy effects on cultured human gingival fibroblasts proliferation using different irradiance and same fluence. Lasers Surg Med, 29, 179 (2001).
Fekrazad, R., et al., The effect of low-level laser therapy (810 nm) on root development of immature permanent teeth in dogs. Lasers Med Sci (2014).
Erdle, B.J., et al., Effects of continuous-wave (670-nm) red light on wound healing. Dermatol Surg, 34, 320 (2008).
Hakki, S.S. and S.B. Bozkurt, Effects of different setting of diode laser on the mRNA expression of growth factors and type I collagen of human gingival fibroblasts. Lasers Med Sci, 27, 325 (2012).
Houreld, N.N., S.M. Ayuk, and H. Abrahamse, Expression of genes in normal fibroblast cells (WS1) in response to irradiation at 660 nm. J Photochem Photobiol B, 130,146 (2014).
Kreisler, M., et al., Low level 809-nm diode laser-induced in vitro stimulation of the proliferation of human gingival fibroblasts. Lasers Surg Med, 30, 365 (2002).
Polimeni, G., A.V. Xiropaidis, and U.M. Wikesjo, Biology and principles of periodontal wound healing/regeneration. Periodontol 2000, 41, 30 (2006).
Hakkinen, L., V.J. Uitto, and H. Larjava, Cell biology of gingival wound healing. Periodontol 2000, 24, 27 (2000).
Sculean, A., R. Gruber, and D.D. Bosshardt, Soft tissue wound healing around teeth and dental implants. J Clin Periodontol, 41 Suppl 15, S6 (2014).
Raja, S., G. Byakod, and P. Pudakalkatti, Growth factors in periodontal regeneration. Int J Dent Hyg, 7,82 (2009).
Pitaru, S., C.A. McCulloch, and S.A. Narayanan, Cellular origins and differentiation control mechanisms during periodontal development and wound healing. J Periodontal Res, 29, 81 (1994).
de Sousa, A.P., et al., Effect of LED phototherapy of three distinct wavelengths on fibroblasts on wound healing: a histological study in a rodent model. Photomed Laser Surg, 28, 547 (2010).
Choi, H., et al., Inflammatory cytokines are suppressed by light-emitting diode irradiation of P. gingivalis LPS-treated human gingival fibroblasts: inflammatory cytokine changes by LED irradiation. Lasers Med Sci, 27, 59 (2012).
Xavier, M., et al., Anti-inflammatory effects of low-level light emitting diode therapy on Achilles tendinitis in rats. Lasers Surg Med, 42, 553 (2010).
Chao, W. and P.A. D’Amore, IGF2: epigenetic regulation and role in development and disease. Cytokine Growth Factor Rev, 19, 111 (2008).
Suh, H.S., et al., Insulin-like growth factor 1 and 2 (IGF1, IGF2) expression in human microglia: differential regulation by inflammatory mediators. J Neuroinflammation, 10, 37 (2013).
Haisa, M., The type 1 insulin-like growth factor receptor signalling system and targeted tyrosine kinase inhibition in cancer. J Int Med Res, 41, 253 (2013).
Flanders, K.C., Smad3 as a mediator of the fibrotic response. Int J Exp Pathol, 85, 47 (2004).
Jinno, K., et al., Acceleration of palatal wound healing in Smad3-deficient mice. J Dent Res, 88, 757 (2009).
Ashcroft, G.S., et al., Mice lacking Smad3 show accelerated wound healing and an impaired local inflammatory response. Nat Cell Biol, 1, 260 (1999).
Krisanaprakornkit, S., et al., CD99 ligation induces intercellular cell adhesion molecule-1 expression and secretion in human gingival fibroblasts. Arch Oral Biol, 58, 82 (2013).
Liu, J., et al., Intracellular adhesion molecule-1 is regulated by porphyromonas gingivalis through nucleotide binding oligomerization domain-containing proteins 1 and 2 molecules in periodontal fibroblasts. J Periodontol, 85, 358 (2014).
Ricard-Blum, S. and F. Ruggiero, The collagen superfamily: from the extracellular matrix to the cell membrane. Pathol Biol (Paris), 53, 430 (2005).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, JT., Hong, K.S. Effect of light-emitting-diode irradiation on the proliferation and migration in human gingival fibroblasts. Tissue Eng Regen Med 12, 37–42 (2015). https://doi.org/10.1007/s13770-014-9061-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-014-9061-7