Abstract
Anaerobic digestion (AD) has become the technology of choice for organic waste treatment as an environmentally beneficial and sustainable waste treatment technology. However, the nitrogen content of these organic waste streams is generally high. Ammonia is produced in the biodegradation of nitrogenous organic matter. Low concentrations of ammonia favour AD, but high concentrations can lead to digestive system failure. To address the issue of ammonia inhibition and ensure the stability of the digestive system, numerous physical, chemical, and biologicalmethods aimed at controlling ammonia levels and/or strengthening the biological processes have been proposedand developed. Literature evidence suggests that differences in AD reaction conditions and microbial sources result in different tolerances of the digestive system to ammonia and nitrogen. This paper summarises and compares the inhibitory effects of ammonia nitrogen under different conditions and the existing regulatory measures to alleviate ammonia nitrogen inhibition. In addition, since the core of the digestive system is microorganisms, this paper explains the mechanism of ammonia stress especially at the microbial level, and in this way, it explores the future direction of research using biofortification. This review provides a theoretical reference for solving the problem of ammonia nitrogen inhibition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As the global population continues to grow and the economy develops at a rapid pace, municipal solid waste (MSW) generation has surged worldwide. Currently, the global generation of MSW is about 2.01 billion tonnes per year (ranging from 0.11 to 4.54 kg/capita/day), but it is projected to increase to 3.40 billion tonnes by 2050 (Rosas-Mendoza et al. 2024). Most of this MSW is openly wasted and very little of it is converted into useful resources. An estimated 93% of waste is either dumped or incinerated, predominantly in less developed countries (Khurram et al. 2024). Organic waste (OW) accounts for 42–69 percent of MSW. These include crop residues, animal manure, landscaping waste, municipal sludge, animal inclusions from slaughterhouses, food waste, etc. (Pongsopon et al.2023; Khurram et al. 2024). Inadequate management of organic waste can lead to various environmental hazards and even threaten human health. It is estimated that about 5% of total greenhouse gas emissions are due to open dumping of OW (Sailer et al. 2021).
How to properly deal with organic waste has become an important issue for human beings to solve at present, the current conventional treatment technology includes measures such as crushing direct discharge, landfill, incineration and so on (Guo et al. 2023) but faces the problem of secondary pollution from siltation and blockage of urban sewage network, leachate leakage and dioxin and greenhouse gas emissions. Anaerobic digestion of organic waste has received widespread attention as an eco-friendly and economically viable method. the technology has been applied to treat a variety of wastes, including municipal sludge, poultry manure, food/fermentation industry wastes, and concentrated municipal wastewater. (Tiwari et al. 2023; Rivera et al. 2023; Paranjpe et al.2023).As of 2017, there were at least 118 kitchen waste treatment projects in China with a scale of 50 t/d or more, of which 76.1% used anaerobic digestion technology. From 2009 to 2020, the number of anaerobic digestion plants in Europe increased from 6,227 to 19,000 (Zhang et al.2023). Simply put, anaerobic digestion relies on the synergistic action of hydrolysing acidifying microorganisms and methanogenic microorganisms, a process that converts organic matter into biogas, biogas residue and a carbon-rich fermentation broth. As the technology continues to mature, it has become a key technology for reducing organic waste, recovering biomass energy, and producing biofuels and energy (O'Connor et al. 2021). Research indicates that AD is highly effective for treating and managing OW (Granzotto et al. 2021).
Ammonia inhibition is a challenging issue in the AD of nitrogen-rich substrates(e.g.food waste and animal waste) and hindersthe energy recovery from organic wastes. Ammonia is produced by the biodegradation of nitrogenous organic matter in organic waste. The low concentrations of ammonia favor AD, but high concentrations of ammonia can lead to digestive system failure (Li et al.. 2023; O'Connor et al. 2023). The two main forms of total ammonia nitrogen (TAN) are ammonium ions (NH4+) and free ammonia (NH3, FAN), both of which can directly or indirectly cause inhibition in AD systems (Lendormi et al. 2022; Mlinar et al. 2022). The relationship between the two is expressed in Eq. (1) (Xiao et al. 2022a). Typically, FAN is considered the primary cause of inhibition because hydrophobic FAN molecules may passively diffuse through cellular membranes, leading to proton imbalance and/or potassium deficiency (Shi et al. 2017). Additionally, FAN enters microbial cells by passive diffusion and is subsequently converted to NH4+ through binding with extracellular protons (H+), resulting in alterations in intracellular pH. To maintain intracellular proton homeostasis, cells actively transport potassium ions out of the cell via energy-consuming potassium pumps in the cell membrane. This process increases the energy required for cellular maintenance and limits certain specific enzymatic reactions(Mlinar et al. 2022; Peng et al. 2023a). Various inhibitory thresholds of total ammonia nitrogen (TAN) concentrations, ranging from 3.4 to 5.77 gL−1, have been reported, resulting in severe methane yield losses in the AD process ranging from 39 to 100% (Li et al. 2023). These outcome variations can be attributed to differences in temperature, reactor configuration, and the microbial communities that develop in distinct systems [Li et al. 2023].
Recent studies have focused on regulatory strategies to mitigate ammonia inhibition during anaerobic digestion (AD) of nitrogenous organic wastes. Various physical, chemical, and biological methods have been developed to control ammonia levels and enhance biological processes. Examples include substrate dilution, adjustment of the carbon to nitrogen ratio, pH control and ammonia recovery through membrane distillation, in addition, other methods have been tested to enhance the biological process including bioaugmentation and domestication(Jo et al. 2022; Wang et al. 2023); addition of various support materials such as activated carbon and magnetite (Li et al. 2023); and provision of trace elements and use of blowdown processes (Meng et al. 2020; Pedizzi et al. 2017). However, few articles have discussed and summarised the mechanism of ammonia inhibition in the AD process of nitrogenous organic wastes at the microbial level. Fundamentally, the AD process is a multi-stage, multi-level biochemical process that is mainly influenced by microorganisms, which are the core of the AD system(Li et al. 2017). It has been found that certain microorganisms are resistant to ammonia nitrogen inhibition during AD. However, coherent and targeted regulatory mechanisms to alleviate ammonia nitrogen inhibition remain elusive due to the complexity and variability of substrate properties, microbial sources and reaction conditions. To address ammonia inhibition and ensure digestive system stability, it is imperative to understand the extent of ammonia inhibition under various conditions, investigate the mechanisms and patterns of ammonia inhibition, and develop potential future regulatory strategies (Qian et al. 2017).
Based on this, this paper firstly describes the ammonia inhibition under different temperature, pH and reactor conditions. And the study of process intensification to implement ammonia inhibition mitigation in AD systems is described in detail. In addition, since the digestion process is mainly dominated by microorganisms, this paper focuses on analysing the causes and patterns of ammonia inhibition in AD from a microbial perspective. Finally, this paper summarises the current strategies and measures for mitigating ammonia inhibition in AD of nitrogenous organic wastes and looks forward to future research directions. This review aims to provide theoretical guidance for mitigating ammonia inhibition in AD.Fig. 1 describes the structure of this review.
Inhibitory effect of ammonia nitrogen on AD process under different conditions
Although ammonia nitrogen promotes the growth of microorganisms at certain concentrations, it can exceed a threshold during substrate degradation and become toxic to microorganisms. Specifically, Anaerobic microorganisms are favorable to AD at ammonia concentrations of 50 ~ 200 mg/L, experience no antagonistic effects at 200 ~ 1000 mg/L, and are inhibited at 1500 ~ 3000 mg/L, especially in high pH anaerobic systems. Moreover, When ammonia nitrogen exceeds 3000 mg/L, microorganisms are inhibited to varying degrees in the AD process under any pH condition (Sung et al. 2003; Procházka et al. 2012b). However, differences in temperature, reactor configuration, and the ammonia nitrogen inhibition levels tolerated by microbial communities in different systems make it impossible to accurately define thresholds. Table 1 lists the extent of ammonia nitrogen inhibition by each type of substrate under different conditions (Alsouleman,K 2019).
Different reactor types
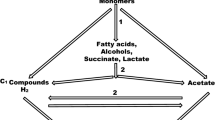
Conventional AD systems are mainly categorized into single-phase and two-phase systems. In single-phase AD systems, all four AD steps occur concurrently within one reactor, while in two-phase AD systems, the acidification and methanization phases are separated into two tandem reactors (Ren et al. 2018; Shen et al. 2013), thus providing suitable conditions for the survival of acid-producing bacteria and methanogenic archaea. However Single-phase reactors lead to an increase in system loading because each reaction occurs in the same reactor. The rapid degradation of nitrogen-containing organic matter in a short period results in a swift increase in ammonia nitrogen content, which inhibits the activity of methanogenic bacteria and affects subsequent reactions (Bouallagui et al. 2009). For the same reactor, different feeding methods result in varying resistance to ammonia inhibition. For instance, Tian et al. (2017) evaluated three reactors (batch, fed-batch, and continuous) operating at medium (37 °C) and high (55 °C) temperatures and found that the batch-fed reactor had twice the free ammonia concentration of the batch reactor and that the continuously stirred reactor was inhibited at lower ammonia levels. In contrast, The two-phase reactor has the advantage of buffering the load in the first stage, allowing a more stable feed rate into the second stage for methane production. Ding et al. (2021) investigated the feasibility of a two-stage system to digest high-solids food waste as the sole feedstock in long-term operation. Compared to a single-stage system, the two-stage system had a 33.3% increase in food waste load, an 18.2% increase in energy yield, and was more resistant to ammonia nitrogen inhibition. However, the increase in system load can also lead to instability in the two-phase system. Ganesh et al. (2014a) conducted a comparative study between single-phase and two-phase digestion of fruit and vegetable wastes and found that the two-phase system showed instability with lower methane and energy yields when the system load reached 7.0 kg VS/m3 d. Since high concentrations of ammonia nitrogen can inhibit the digestion system and limit its organic load, future research should focus on reactor design to increase the organic load and avoid ammonia nitrogen inhibition (Nasr et al. 2012; Shen et al. 2013; Christou et al. 2021; Adghim et al. 2022). Figure 2 depicts the suppression of ammonia nitrogen under different conditions.
Different pH
The pH of an anaerobic fermentation system affects both microbial activity and ammonia nitrogen concentration. Under normal reactor operation, if the digested substrate is mainly composed of proteins, the pH of the digestion reactor is generally high. However, When the system pH is elevated, the transition of ammonia nitrogen to free state NH3 will further affect the activities of anaerobic microorganisms, thus causing the accumulation of volatile fatty acids (VFAs) (Zhang et al. 2018; Park et al. 2018). At pH 6.5–8.5, the free ammonia content in the system increases by 3–18 times with a pH increase of 0.6–1.3. Consequently, after the accumulation of VFAs exceeds the buffer threshold, the system's pH will decrease significantly, resulting in system destabilization (Tian et al. 2019). For example, Ho et al. (2012) increased the biogas production of the system from 200 mL/L at pH 8.3 to 680 mL/L at pH 6.5 by adjusting the pH of the AD reactor feed, a 2.4-fold increase. Therefore, controlling the system pH is key to alleviating ammonia and nitrogen inhibition and maintaining the smooth operation of AD.
Different temperatures
Temperature is a significant factor affecting the ammonia threshold of the digestion system, as it isdirectly related to the microbial growth rate and free ammonia concentration in the digestion system (Ye et al. 2022; Liu et al. 2024). Medium and high temperature digestion each have their own advantages and disadvantages. Specifically, medium-temperature AD is cost-effective, has slow ammonia nitrogen accumulation, and higher biogas purity, but the biogas production rate is slower, and the tolerance limit for ammonia nitrogen is lower than that of high temperature digestion. Additionally, it also cannot effectively kill pathogens in the digestive system. High-temperature digestion is faster and more productive due to more complete degradation of raw materials, and the high temperature can effectively kill pathogens in the system, which is conducive to the secondary utilization of digestate. However, the methane content of the gas produced is lower, and ammonia nitrogen accumulates faster, leading to inhibition. Kim et al. (2011) found that the degree of protein destruction was higher under thermophilic conditions, and ammonia nitrogen content was higher in thermophilic phases due to protein degradation at increased organic loading rates (OLR). Furthermore, when the temperature increases, it enhances microbial metabolism, and the free ammonia content in the system rises consequently, increasing the ammonia nitrogen content in the system (Massé. et al. 2014; Angelidaki and Ahring 1993). For high ammonia loading digesters, the low-temperature AD process tends to have less ammonia inhibition and is found to be more stable than high/moderate temperatures. High-temperature digestion (operating temperature: 50 °C ~ 55 °C) is more susceptible to ammonia inhibition than moderate temperature digestion (operating temperature: 35 °C ~ 40 °C), leading to an unstable digestion system (Montecchio et al. 2017). Li et al. (2022) investigated the effects of bio-reinforcement on ammonia nitrogen in the digestive system at moderate and high temperatures using sludge as the substrate to explore the mitigation of ammonia inhibition by bio-augmentation. The results showed that methane production decreased by about 21% and 28% under medium and high temperature conditions, respectively, and thermophilic microorganisms responded more strongly to ammonia inhibition.
Microbial domestication
Different inoculated microorganisms have varying levels of tolerance to ammonia nitrogen, and microorganisms domesticated with high concentrations of ammonia nitrogen can improve their resistance to it. Studies have shown (Yenigün and Demirel 2013; Christou et al. 2021) that domestication of the microbial community in AD systems by progressively increasing the ammonia levels can increase the community's tolerance to ammonia. Specifically, inoculation with undomesticated microorganisms completely inhibited the digestion process when the system TAN concentration reached1700 ~ 1800 mg/L, while inoculation with domesticated microorganisms increased the inhibition threshold of TAN concentration to 5000 mg/L. Corresponding to a FAN concentration of 256 mg/L, the acid production process remained stable, indicating that the digester could still operate stably under low methane production conditions. However, Complete inhibition was observed when the TAN concentration reached 6700 mg/L. The effectiveness of biofortification is significantly influenced by the microbial composition of consortia. Wang et al. (2023) obtained two microbial consortia (syntrophic microbial consortium, MC, and hydrogenotrophic methanogen consortium, SS) by pure culture domestication and applied them to a nitrogen-enriched AD system (TAN concentration > 8 g/L). The results indicated that AD performance was restored within 21 days for the MC treatment and 83 days for the SS treatment. Although domestication of microorganisms is an effective method for resisting ammonia inhibition, it should be noted that domestication at high ammonia concentrations may cause irreversible damage to the microbial community structure (Nie et al. 2015; Zhang et al. 2022; Poirier et al. 2017).
To summarize, in the practical application of AD, two-phase reactors stand out due to their strong buffering capacity. Nevertheless, when the system load continuously increases, two-phase reactors can be inhibited. In engineering applications, the system load can be equalized by choosing an intermittent feeding method to ensure stable reactor operation. The selection of temperature and pH should focus on the appropriate range for microorganisms in the digestive system; Specifically, medium and high temperatures enhance microbial activity, thereby increasing the free ammonia concentration in the system, which leads to higher ammonia–nitrogen concentrations. Furthermore, changes in pH indicate variations in ammonia nitrogen and volatile acid concentrations in the system. Therefore, to prevent ammonia nitrogen inhibition, pH adjustment should be carried out based on low-temperature digestion. To further enhance the system's tolerance to ammonia nitrogen, inoculation with microorganisms that have been domesticated with high ammonia nitrogen concentrations can be considered. Within a certain range, as the ammonia nitrogen concentration in the system gradually increases,microorganisms can adapt to higher ammonia nitrogen environments, thus resisting ammonia nitrogen inhibition.
Ammonia Stress Mechanism
High ammonia nitrogen concentrations are an important factor contributing to the instability of AD. The bacterial flora is closely related to the operational efficiency and methane production rate of AD. The system includes hydrolysis-acidification bacteria, hydrogen-producing acetogens, acetotrophic methanogenic archaea, and hydrogenotrophic methanogenic archaea (Yang et al. 2018; Tian et al. 2018b). Specifically, high ammonia concentrations affect the structure of the bacterial population in the AD system, thereby reducing the efficiency of AD. For instance, it has been demonstrated that the microbial community within the AD system responds to high concentrations of ammonia nitrogen, with methanogens being more sensitive to ammonia stress than hydrolysis-acidification bacteria and hydrogen-producing acetogens. Under nitrogen stress, the pathway of methane production is altered, and the dominant community in the system shifts from acetotrophic methanogenic archaea to hydrogenotrophic methanogenic archaea (Wang et al. 2022). Figure 3 shows the mechanism of ammonia inhibition in the AD system.
Effect of ammonia on VFAs accumulation in AD systems
When the anaerobic system is destabilized by ammonia inhibition, both inhibition of acid secretion and the accumulation of large amounts of short-chain fatty acids, such as propionic acid and butyric acid, are often observed. However, the inhibitory effect of high nitrogen stress on acetogenic bacteria is selective, affecting the overall abundance of acetogenic bacteria such as digestive Enterobacteriaceae of the genus Pelotomaculum, desulphurizing Enterobacteriaceae of the genus Desulfotomaculium, and acid-producing hydroxyacetic acid bacteria of the genus Pelotomaculum. High nitrogen stress also resulted in a reduction in the genera Syntrophomonas and Syntrophus, as well as the overall abundance of the phylum Ascomycetes. The relative abundance of Desulfovibrio, a phylum of Proteobacteria, has also decreased. According to the literature, propionate and butyrate are directly utilized by Desulfovibrio, which converts them into acetate (Chen et al. 2016). Therefore, high ammonia concentrations significantly reduce the abundance of propionate and butyrate oxidizing microorganisms and their accessory bacteria.
Changes in the efficiency of the AD process and microbial population dynamics in food waste were examined by Peng et al. (2018). The study found that higher ammonia nitrogen concentrations significantly reduced Methanosaeta species abundance and inhibited acetic acid metabolism in the AD system. The accumulation of acetic acid inhibits the degradation of other volatile fatty acids, such as propionic acid and valeric acid, leading to a complete disruption of the entire AD metabolic network and possibly the collapse of the AD reactor. Niu et al. (2015a) found that high ammonia concentration increased the diversity of bacterial communities and enriched acid-producing bacteria. Furthermore, throughout the entire AD process, hydrogenotrophic methanogenic archaea predominated, and the lack of acetotrophic methanogenic archaea led to the accumulation of acetic acid and other volatile fatty acids. This accumulation resulted in a reduction in methane production and destabilization of the reactor. It is evident that hydrogenotrophic methanogenic archaea are more resistant to ammonia stress than acetotrophic methanogenic archaea, and blocking acetic acid metabolism is the main cause of AD system destabilization (Ziganshin et al. 2013). In addition, Among the microorganisms that metabolize acetic acid, Methanosaeta is the most sensitive to ammonia, with its activity inhibited when TAN exceeds 2000 mg/L. In contrast, Methanosarcina and syntrophic acetate-oxidizing bacteria (SAOB) are more tolerant to ammonia. Thus,the introduction of domesticated Methanosarcina and SAOB into the system can restore system stability.
Effects of ammonia nitrogen on the dominant bacterial species in the AD system
In recent years, many researchers have attempted to introduce ammonia–nitrogen interference into reactors to study the microbial community structure, dominant bacterial species, and metabolic functions of AD flora under nitrogen stress conditions. For example, Ruiz-Sánchez et al. (2018) studied bacteria and archaea in AD systems under ammonia stress. They assessed diversity and found that ammonia had different effects on microbial diversity in different groups and conflicting effects on microbial diversity for each metabolic function. By comparing differences in the number of dominant microorganisms in AD reaction systems operating at four ammonia concentrations, they found that the dominant acidifying hydrolysing bacteria were from the genera Sporocytophaga and Nitratalea in AD reaction systems with TAN < 3000 mg/L. Similarly, Buhlman et al. (2019) showed that the dominant methanogenic pathway shifted from acetotrophic methanogens to synthetic trophic acetate oxidative-hydrogenotrophic methanogens when ammonia nitrogen concentration increased to 6133–8366 mg/L (Wang et al. 2015; Lee et al. 2021). In addition, the results of Niu et al. (2015a) showed that bacterial communities subjected to ammonia inhibition mainly exhibited resistant or redundant traits. During ammonia inhibition, the dominant bacterial species were replaced to ensure that the gas production efficiency of the anaerobic digestion (AD) system was restored.
Influence of ammonia nitrogen on the activity of intracellular enzymes of microorganisms
During the AD process, the biotransformation of organic substrates is facilitated by various enzymes within microbial cells, as illustrated in Fig. 3. Recent studies have increasingly focused on the role of microbial enzymes during anaerobic fermentation under ammonia stress. Using integrated multi-omics analysis, Zhang et al. (2022) demonstrated that ammonia stress in the AD system significantly reduces the expression activity of methyl coenzyme M reductase in methanogenic filamentous bacteria, thereby inhibiting the conversion of acetate to methane (Yan et al. 2020). In addition, Ammonia also inhibits the methylmalonyl-CoA (MMC) pathway in Pelotomaculum by suppressing the expression of succinyl coenzyme A synthetase, leading to the inhibition of propionate oxidation. Acetic acid metabolism is particularly sensitive to ammonia stress in AD. High ammonia nitrogen concentrations inhibit the expression of methyl coenzyme M reductase in methanogenic filamentous bacteria, thereby inhibiting acetic acid metabolism. However, continuous ammonia stress shifts the dominant acetate-degrading microorganisms to methanotrophic octococci with higher ammonia tolerance. The microbial community can be continuously optimized to restore acetate metabolism through the acetate-methane (AM) pathway, facilitated by enzymes such as acetic acid kinase, phosphoacetyltransferase, and deaminase (Poirier et al. 2016).
Under ammonia stress, propionic acid accumulates in the system, significantly reducing the rate of methane production. The abundance of propionate-oxidizing bacteria (SPOB) decreases, along with the expression of methane-related enzymes that degrade propionate, indicating that propionate metabolism is highly inhibited and more sensitive to ammonia stress. Therefore, SPOBs are crucial in influencing the function of the AD system under ammonia stress. They inhibit propionate metabolism by suppressing the expression of succinyl coenzyme A synthetase and the conversion of methylmalonyl coenzyme A. Wang et al. (2023) found that the accumulation of short-chain fatty acids, such as propionic acid, affects the activity of methanogenic bacteria, indirectly leading to system destabilization. Excess free ammonia reduces the number and activity of filamentous methanogenic bacteria of the genus Methanosaeta, inhibiting acetate metabolism and leading to reactor destabilization. Excess long-chain fatty acids (LCFAs) in the system create a synergistic inhibitory effect with ammonia. The relative abundance of Petrimonas and Paraclostridium species decreases, suggesting that high ammonia concentrations inhibit the β-oxidation of LCFAs (Wu et al. 2019). The abundance of enzymes such as acetate kinase, phosphopyruvate acetyltransferase, pyruvate synthase, phosphopyruvate hydratase, phosphoglycerate dehydrogenase, and glycine hydroxymethyltransferase, which are associated with acetic acid dehydroxylation, is significantly reduced. This suggests that high concentrations of nitrogen and ammonium primarily inhibit the oxidation reaction step of methane under synergistic inhibition conditions (Capson-Tojo et al. 2020; Peng et al. 2023b; Yu et al. 2021).
Effect of ammonia on microbial cell morphology
The effect of free ammonia on AD performance was investigated by Calli et al (2005).They found that the cluster structure of Methanosarcina Methanoctococcus spp. was significantly decomposed at free ammonia concentrations greater than 700 mg/L. Furthermore, scanning electron microscope observations revealed obvious wrinkles, deformations, and cracks on the surface of the cells, indicating the destruction of cellular integrity (Park et al. 2018). In contrast, cells in the blank control group were intact in shape, tightly packed, and had smooth surfaces. The breakdown of cellular integrity leads to the loss of protective functions, which in turn results in cell damage, lysis, and death (Yan et al. 2019). Therefore, this study suggests that the effect of high concentrations of ammonia nitrogen on microbial cell activity and morphological structure is a crucial factor in ammonia inhibition. Different species of methanogenic bacteria exhibit varying tolerance to ammonia nitrogen, likely due to differences in cell morphology. For instance, filamentous cells with a larger specific surface area, characteristic of acetotrophic methanogenic archaea of the genus Methanosaeta, allow free ammonia to diffuse more easily into the cell. On the other hand, Methanosarcina and some species of hydrogenotrophic methanogenic archaea can form cell clusters under high ammonia stress, creating ecological niches in the centers of these clusters that help resist ammonia toxicity.
Effect of ammonia nitrogen on lipid molecules of microbial cells
The cell membrane is the main route for the exchange of substances between the cell and its environment and is crucial for maintaining growth and proper function. During the AD process, organic substrates generally need to be transported into the cell by crossing the cell membrane to metabolize substances (Linda-M et al. 2001). In recent years, researchers have shown interest in the effects of high ammonia nitrogen exposure on the key properties of microbial cell membranes. For example, Liu et al. (2023) showed that the permeability of the acetotrophic methanogenic archaea cell membrane increased significantly under nitrogen stress, exhibiting significant depolarization of the membrane potential, as shown in Fig. 4. Furthermore, fluorescence polarization detection was conducted, revealing that the fluorescence polarization of cells in the AD system under high concentrations of ammonium nitrogen was significantly higher than in the control group (Tian et al. 2018a; Astals et al. 2021). These results indicate that high concentrations of ammonium nitrogen cause a significant decrease in cell membrane fluidity.
Controlling strategy
During the AD process, approximately 33–80% of nitrogenous organic matter, such as urea, proteins, amino acids, and nucleic acids, are hydrolyzed and fermented, releasing ammonia and nitrogen as end products. Ammonia inhibits the normal metabolic functions of anaerobic microorganisms by disrupting intracellular pH/proton and potassium (K+) ion balances, exacerbating cellular energy depletion, and inhibiting the activities of specific enzymes associated with methanogenic metabolism. Furthermore, due to the heightened sensitivity of methanogenic bacteria to ammonia, it leads to the accumulation of volatile fatty acids, thereby exacerbating the deterioration of the AD process. Strategies to mitigate ammonia inhibition by adjusting operating parameters (e.g., temperature, pH, and carbon to nitrogen ratio) and employing physicochemical methods (e.g., dilution, precipitation, air stripping, membrane separation, and ion exchange) have been extensively studied and reviewed. (Xu et al. 2022) Table 2 lists several measures to mitigate ammonia inhibition in the AD process.
Adjustment of the C/N ratio
The adjustment of the carbon to nitrogen (C/N) ratio is considered a crucial measure in mitigating the inhibition caused by ammonia nitrogen during the AD of organic wastes. When the C/N ratio is too low, it can lead to the accumulation of ammonia nitrogen within the system, potentially inhibiting AD microorganisms (Capson-Tojo et al. 2020; Yang et al. 2022). Conversely, a fermentation feedstock with an excessively high C/N ratio may result in an inadequate nitrogen source within the system, leading to an underutilized carbon source. The combined AD of various organic wastes is recognized for its ability to enhance waste utilization efficiency and reduce the need for multiple treatment facilities, thereby resulting in cost savings (Peng et al. 2022). Moreover, it is acknowledged that this process aids in improving the stability of the digestion procedure by increasing the C/N ratio of the feedstock, effectively mitigating the effects of ammonia nitrogen inhibition. In a study conducted by Wang et al. (2022), it was observed that when kitchen waste and food waste were co-digested at a mass ratio of 2:1, there was a significant increase in the gas production rate and methane content compared to the AD of kitchen waste alone. Specifically, at an organic loading rate (OLR) of about 120 kg·d-1, the average volumetric mean biogas production rates for the AD and co-digestion of the two substrates were measured at 2.02, 0.75, and 2.3 m3·m-3·d-1, respectively. Similarly, the average methane content in the produced biogas was recorded at 38.4%, 21.2%, and 63.8% for the AD of kitchen waste alone and the co-digestion of the two substrates, respectively (Mahdy et al. 2020). Furthermore, Beniche et al. (2021) observed promising outcomes from the co-digestion of food waste combined with leaves and stems of kale and cauliflower at a C/N ratio of 45. The resultant mixed substrate exhibited high biodegradability, reaching 98%. This co-digestion process yielded a methane production of 475 mL STP CH4/g VS, both of which displayed enhancements compared to the performance achieved through sole AD.
Selection of adapted microorganisms
Microbial domestication is a pivotal strategy in alleviating ammonia nitrogen inhibition within waste digestion systems. This approach involves the deliberate cultivation or introduction of microbial flora capable of tolerating elevated ammonia nitrogen concentrations, thereby significantly enhancing the system's ability to manage ammonia nitrogen effectively. An essential phase in the domestication process is the screening and cultivation of microorganisms resilient to high ammonia nitrogen concentrations (Chen et al. 2018). This is typically achieved by subjecting the microbial flora to gradually increasing levels of ammonia nitrogen. Jo et al. (2022) demonstrated the enhancement of methane production rates within the system, increasing from 154.6 ± 9.9 mL/g COD to 269.6 ± 3.6 mL/g COD through microbial domestication. This domestication led to observable alterations in both bacterial and archaeal populations. Notably, the transition of archaeal populations from Methanobacterium spp. to Methanosaeta spp. and Methanosarcina spp. occurred concurrently throughout the domestication process (Carballa et al. 2015; Peng et al. 2023c). Wang et al.(2023) applied two microbial communities (MC and SS) through domestication to a nitrogen-enriched AD system and found that the MC and SS treatments restored AD performance within 21 and 83 days, respectively. Analysis of the 13C isotope indicated that both MC and SS enhanced the hydrogenotrophic pathway.
Ammonia removal
The accumulation of ammonia nitrogen can significantly impede the efficiency of AD and potentially cause process failure. Ameliorating ammonia nitrogen inhibition can be accomplished through several methods, including the addition of auxiliary materials such as clay, zeolite, and guano, as well as utilizing membrane reaction contactors, incorporating trace elements, and employing the blow-off method. For instance, the guano stone method involves leveraging magnesium and phosphorus within guano to create insoluble magnesium ammonium phosphate precipitation (Ye et al. 2024; Zhuo et al. 2018). This process effectively removes phosphorus and aids in denitrification, thereby mitigating ammonia nitrogen accumulation. Li et al. (2022) investigated the impact of guano stone precipitation in removing ammonia nitrogen from the anaerobic fermentation process of chicken manure. Their study revealed a significant reduction in the ammonia nitrogen concentration within the test group reactor, decreasing from 2,937 mg/L to 1,466 mg/L. Consequently, the average methane production improved by 18%, increasing to 0.39 L/g compared to the control group's 0.33 L/g. Furthermore, the addition of trace element Fe demonstrated an antagonistic effect on ammonia nitrogen, with this effect notably intensifying as the ammonia nitrogen concentration increased. Meng et al. (2020) explored the use of zero valent iron (ZVI, 150 µm) to enhance methanogenic capacity. The introduction of ZVI at 160 mM notably amplified cumulative methane production by 22.2% and further reduced the high-solid anaerobic digestion (HSAD) duration by 50.6%. Additionally, the blow-off method, which transfers ammonia nitrogen from the liquid phase to the gas phase, emerged as an efficient physical nitrogen removal process with low investment costs, relatively straightforward equipment requirements, and simple operational procedures. Pedizzi et al. (2017) implemented an air sidestream vapor stripping process to reduce ammonia nitrogen concentration. Their study demonstrated a successful reduction of ammonia nitrogen concentration from 2.4 ± 0.1 g N-TAN L-1 to 1.1 ± 0.1 g N-TAN L-1, without compromising process stability. Furthermore, they achieved a reduction from 4.5 ± 2.0 g N-TAN L-1 to 2.0 ± 0.1 g N-TAN L-1, highlighting the effectiveness of their approach. Similarly, Zhuang et al. (2018) demonstrated that the addition of magnetite nanoparticles resulted in a 36–58% increase in methane production compared to the control group. Additionally, it was observed that magnetite nanoparticles had a minimal impact on TAN concentration, suggesting that conductive materials have a relatively minor effect on ammonia levels, yet they diminish the inhibitory effects of ammonia nitrogen (Ngo et al. 2023; Provolo et al. 2017; Zhao et al. 2019).
limitations and future directions of research
Based on the above, the anaerobic digestion (AD) process is a complex biochemical system grounded in thermodynamic principles and driven by microorganisms that work synergistically through commensal linkages to maintain system stability. However, these microorganisms are highly sensitive to environmental fluctuations, which, given the complexity of the substrates and the stochastic nature of AD operating conditions, could adversely affect the performance of AD facilities. Consequently, there has been significant scientific interest in exploring whether the addition of microorganisms with specific biodegradative capabilities—a bioaugmentation strategy involving the introduction of specialized microbial functional groups into inhibited AD systems—can mitigate toxicity under high ammonia concentrations and enhance reactor performance. For instance, Methanoculleus and Methanosarcina have been identified as effective bioaugmentation agents for counteracting ammonia inhibition. Research has shown that adding Methanoculleus to an AD system utilizing municipal solid waste as a substrate can increase methane production by 21% (Wang et al. 2023). Additionally, the introduction of bacteria-rich bioadditives, such as propionic acid and butyric acid-degrading bacteria, has been found to accelerate the conversion of volatile fatty acids (VFAs) to methane. This acceleration is largely attributed to the interactions within the complex bacterial community, which help reduce hydrogen partial pressure (Li et al. 2022).
Although the bioaugmentation strategy of cultivating pure strains can be effective in enhancing the digestive performance of anaerobic reactors under ammonia stress, it is accompanied by some risks, i.e., it is difficult for a single archaea to colonise and rebuild the microbial community in systems suppressed by high ammonia and nitrogen concentrations (Wang et al. 2023). In addition, the cost and technical requirements (sterile environment, culture media) associated with the cultivation of pure strains are realities that have to be taken into account. Therefore, biofortification strategies using mixed microbial consortia (consisting of microorganisms that can tolerate inhibitory factors) are a more effective alternative in order to better fit the host microbial community as well as to improve the tolerance of the system to ammonia stress. For example, Yang et al. (2019) combined Methanobrevibacter and syntrophic acetate oxidizing bacteria (SAOB) (Syntrophaceticu schinkii) as a microbial consortium and methane yield was improved by 71%. However, whether these selected microbial consortia are stably able to function in high-ammonia inhibition systems needs to be confirmed by extensive experiments. For instance, Westerholm et al. (2012) combined some SAOBs (Clostridium ultunense sp,Tepidanaerobacter acetatoxydans, and Syntrophaceticus schinkii) with ahydrogenotrophic methanogen (Methanoculleus) to construct a microbial consortium as a biological additive but the digestion performance was not improved after adding the microbial consortium. Interestingly, microbial symbionts obtained through purposeful domestication are more closely related yet better adapted than artificial microbial symbionts. Consequently, they may yield superior results in enhancing digestive performance in AD systems with high TAN concentrations (Wang et al. 2023). However, there remains a knowledge gap regarding the effectiveness and potential mechanisms of microbial symbionts acquired through purposeful domestication as bioadditives for alleviating ammonia inhibition. Specifically, the potential mechanisms of microbial symbionts obtained through purposeful domestication in mitigating ammonia inhibition may be more complex than those of individual archaea or artificially assembled microbial symbionts, and the roles of individual members within a microbial symbiont in mitigating ammonia inhibition are still unknown. Much future research is needed. In addition, a reliable set of mathematical models to predict the efficiency of different biofortification would provide a more efficient solution for selecting a mixed microbial consortium that is tolerant to ammonia stress and thus improve the digestive performance of the AD system.
Conclusion
AD stands as a pivotal biological treatment method that effectively converts organic wastes into valuable biogas while simultaneously reducing waste volume. Nevertheless, the accumulation of ammonia nitrogen within this process can trigger ammonia nitrogen inhibition, thereby restricting the efficiency and stability of the digestion process. To mitigate this inhibition, various measures have been implemented. These include the adjustment of the C/N ratio, pH regulation, addition of VFAs, utilization of ammonia nitrogen adsorbents, adoption of ammonia nitrogen removal processes, alongside gas stripping and blow-off techniques. In essence, these collective measures collectively contribute to the reduction of ammonia–nitrogen concentrations and serve to alleviate the inhibition effects within the AD system. Adjusting the C/N ratio through co-elimination methods aids in diminishing the accumulation of ammonia nitrogen. pH regulation and the utilization of ammonia nitrogen adsorbents assist in preserving a neutral or alkaline environment, thereby reducing the concentration of free ammonia nitrogen. Additionally, vapor stripping and blow-off techniques work to facilitate the release of ammonia nitrogen by enhancing the rate of gas–liquid mass transfer. These approaches collectively enhance the efficiency and stability of the AD system.
In the future, there is potential for further exploration of novel methods and advanced technologies aimed at more effectively mitigating ammonia nitrogen inhibition. Particularly, the investigation of innovative microbial domestication strategies holds promise in enhancing microbial adaptation within high ammonia–nitrogen environments. Moreover, delving into the intricate interrelationships between ammonia nitrogen inhibition and various other waste treatment parameters can significantly contribute to the refinement and optimization of waste digestion systems. Ultimately, such endeavors are poised to significantly enhance the feasibility of mitigating ammonia nitrogen inhibition, thus fostering improvements in the efficiency and stability of the AD process.
Abbreviations
- MSW:
-
Municipal solid waste
- OW:
-
Organic waste
- AD:
-
Anaerobic digestion
- TAN:
-
Total ammonia nitrogen
- FAN:
-
Free ammonia
- OLR:
-
Organic loading rates
- MC:
-
Syntrophic microbial consortium
- SS:
-
Hydrogenotrophic methanogen consortium
- SAOB:
-
Syntrophic acetate-oxidizing bacteria
- WL:
-
Wood-Ljungdahl pathway
- MMC:
-
Methylmalonyl-CoA
- AM:
-
Acetate-methane
- SPOB:
-
Propionate-oxidizing bacteria
- LCFAs:
-
Long-chain fatty acids
- VFAs:
-
Volatile fatty acids
- C/N:
-
Carbon to nitrogen
- OLR:
-
Organic loading rate
- ZVI:
-
Zero valent iron
- HSAD:
-
High-solid anaerobic digestion
- SAOB:
-
Syntrophic acetate oxidizing bacteria
References
Adghim M, Sartaj M, Abdehagh N (2022) Post-hydrolysis ammonia stripping as a new approach to enhance the two-stage anaerobic digestion of poultry manure: Optimization and statistical modelling. J Environ Manage 319:115717. https://doi.org/10.1016/j.jenvman.2022.115717
Adghim M, Sartaj M, Abdehagh N, Strehlar B (2023) Post-hydrolysis versus side-stream ammonia stripping in semi-continuous two-stage anaerobic digestion of high nitrogen feedstock. Waste Manage 168:74–82. https://doi.org/10.1016/j.wasman.2023.05.041
Alsouleman K (2019) Effect of increasing amounts of ammonium nitrogen induced by consecutive mixture of poultry manure and cattle slurry on the microbial community during thermophilic anaerobic digestion. J Microbio Biotechnol 29(12):1993–2005
Angelidaki I, Ahring B (1993) Thermophilic anaerobic digestion of livestock waste: the effect of ammonia. Appl Microbiol Biotechnol 38:560–564. https://doi.org/10.1007/BF00242955
Astals S, José Chávez-Fuentes J, Capson-Tojo G, Hutňan M, Jensen PD (2021) The interaction between lipids and ammoniacal nitrogen mitigates inhibition in mesophilic anaerobic digestion. Waste Manage 136:244–252. https://doi.org/10.1016/j.wasman.2021.10.015
Beniche I, Hungría J, El Bari H, Siles J, Chica A, Martín M (2021) Effects of C/N ratio on anaerobic co-digestion of cabbage, cauliflower, and restaurant food waste. Biomass Conversion Biorefinery 11:2133–2145. https://doi.org/10.1007/s13399-020-00733-x
Bouallagui H, Lahdheb H, Romdan EB, Rachdi B, Hamdi M (2009) Improvement of fruit and vegetable waste anaerobic digestion performance and stability with co-substrates addition. J Environ Manage 90:1844–1849. https://doi.org/10.1016/j.jenvman.2008.12.002
Buhlmann CH, Mickan BS, Jenkins SN, Tait S, Kahandawala TK, Bahri PA (2019) Ammonia stress on a resilient mesophilic anaerobic inoculum: methane production, microbial community, and putative metabolic pathways. Biores Technol 275:70–77. https://doi.org/10.1016/j.biortech.2018.12.012
Calli B, Mertoglu B, Inanc B, Yenigun O (2005) Methanogenic diversity in anaerobic bioreactors under extremely high ammonia levels. Enzyme Microb Technol 37:448–455. https://doi.org/10.1016/j.enzmictec.2005.03.013
Capson-Tojo G, Moscoviz R, Astals S, Robles Á, Steyer JP (2020) Unraveling the literature chaos around free ammonia inhibition in anaerobic digestion. Renew Sustain Energy Rev 117:109487. https://doi.org/10.1016/j.rser.2019.109487
Carballa M, Regueiro L, Lema J (2015) Microbial management of anaerobic digestion: exploiting the microbiome-functionality nexus. Curr Opin Biotechnol 33:103–111. https://doi.org/10.1016/j.copbio.2015.01.008
Chen H, Wang W, Xue L, Chen C, Liu G, Zhang R (2016) Effects of ammonia on anaerobic digestion of food waste: process performance and microbial community. Energy Fuels 30:5749–5757. https://doi.org/10.1021/acs.energyfuels.6b00715
Chen S, He J, Wang H, Dong B, Li N, Dai X (2018) Microbial responses and metabolic pathways reveal the recovery mechanism of an anaerobic digestion system subjected to progressive inhibition by ammonia. Chem Eng J 350:312–323. https://doi.org/10.1016/j.cej.2018.05.168
Christou ML, Vasileiadis S, Kalamaras SD, Karpouzas DG, Angelidaki I, Kotsopoulos TA (2021) Ammonia-induced inhibition of manure-based continuous biomethanation process under different organic loading rates and associated microbial community dynamics. Biores Technol 320:124323. https://doi.org/10.1016/j.biortech.2020.124323
Ding L, Chen Y, Xu Y, Hu B (2021) Improving treatment capacity and process stability via a two-stage anaerobic digestion of food waste combining solid-state acidogenesis and leachate methanogenesis/recirculation. J Clean Prod 279:123644. https://doi.org/10.1016/j.jclepro.2020.123644
Ganesh R, Torrijos M, Sousbie P, Lugardon A, Steyer JP, Delgenes JP (2014) Single-phase and two-phase anaerobic digestion of fruit and vegetable waste: comparison of start-up, reactor stability and process performance. Waste Manage 34:875–885. https://doi.org/10.1016/j.wasman.2014.02.023
Gao Y, Fang Z, Liang P, Zhang X, Qiu Y, Kimura K, Huang X (2019) Anaerobic digestion performance of concentrated municipal sewage by forward osmosis membrane: focus on the impact of salt and ammonia nitrogen. Biores Technol 276:204–210. https://doi.org/10.1016/j.biortech.2019.01.016
Granzotto F, Aita C, Silveira DD, Mayer FD, Pujol SB, Pinas JAV, Hoffmann R (2021) Use of anaerobic biodigestor in the treatment of organic waste from a university restaurant. J Environ Chem Eng 9(5):105795
Guo Y, Liu C, Yin LX, Zhang XX, Shan YQ, Duan PG (2023) Preparation of supercapacitor carbon materials from food waste via low-temperature pyrolysis. J Anal Appl Pyrol 2023(170):105880. https://doi.org/10.1016/j.jaap.2023.105880
Ho L, Ho G (2012) Mitigating ammonia inhibition of thermophilic anaerobic treatment of digested piggery wastewater: use of pH reduction, zeolite, biomass and humic acid. Water Res 46:4339–4350. https://doi.org/10.1016/j.watres.2012.05.016
Jo Y, Cayetano RDA, Kim G-B, Park J, Kim S-H (2022) The effects of ammonia acclimation on biogas recovery and the microbial population in continuous anaerobic digestion of swine manure. Environ Res 212:113483. https://doi.org/10.1016/j.envres.2022.113483
Khurram P, Mansoor Ahammed M (2024) Effect of composition on anaerobic digestion of organic fraction of municipal solid wastes: a review. Bioresour Technol Rep 2024:101777. https://doi.org/10.1016/j.biteb.2024.101777
Kim H-W, Nam J-Y, Shin H-S (2011) A comparison study on the high-rate co-digestion of sewage sludge and food waste using a temperature-phased anaerobic sequencing batch reactor system. Biores Technol 102:7272–7279. https://doi.org/10.1016/j.biortech.2011.04.088
Kizito S, Jjagwe J, Mdondo SW, Nagawa CB, Bah H, Tumutegyereize P (2022) Synergetic effects of biochar addition on mesophilic and high total solids anaerobic digestion of chicken manure. J Environ Manage 315:115192. https://doi.org/10.1016/j.jenvman.2022.115192
Lee J, Kim E, Hwang S (2021) Effects of inhibitions by sodium ion and ammonia and different inocula on acetate-utilizing methanogenesis: Methanogenic activity and succession of methanogens. Biores Technol 334:125202. https://doi.org/10.1016/j.biortech.2021.125202
Lendormi T, Jaziri K, Béline F, Le Roux S, Bureau C, Midoux C, Barrington S, Dabert P (2022) Methane production and microbial community acclimation of five manure inocula during psychrophilic anaerobic digestion of swine manure. J Clean Prod 340:130772. https://doi.org/10.1016/j.jclepro.2022.130772
Li Y, Jin Y, Borrion A, Li H, Li J (2017) Effects of organic composition on mesophilic anaerobic digestion of food waste. Biores Technol 244:213–224. https://doi.org/10.1016/j.biortech.2017.07.006
Li M-T, Rao L, Wang L, Gou M, Sun Z-Y, Xia Z-Y, Song W-F, Tang Y-Q (2022) Bioaugmentation with syntrophic volatile fatty acids-oxidizing consortia to alleviate the ammonia inhibition in continuously anaerobic digestion of municipal sludge. Chemosphere 288:132389. https://doi.org/10.1016/j.chemosphere.2021.132389
Li Z-Y, Inoue D, Ike M (2023) Mitigating ammonia-inhibition in anaerobic digestion by bioaugmentation: a review. J Water Process Eng 52:103506. https://doi.org/10.1016/j.jwpe.2023.103506
Linda M, I.de Poorter, & J. T. Keltjens, (2001) Convenient fluorescence-based methods to measure membrane potential and intracellular pH in the Archaeon Methanobacterium thermoautotrophicum. J Microbiol Methods 47:233–241. https://doi.org/10.1016/S0167-7012(01)00312-8
Liu C, Zhang X, Chen C, Yin Y, Zhao G, Chen Y (2023) Physiological responses of methanosarcina barkeri under ammonia stress at the molecular level: the unignorable lipid reprogramming. Environ Sci Technol 57:3917–3929. https://doi.org/10.1016/j.jwpe.2023.103506
Liu F, Zhang Y, Zhang Y, Yang J, Shen W, Yang S, Quan Z, Liu B, Yuan Z, Zhang Y (2024) Thermodynamic restrictions determine ammonia tolerance of functional floras during anaerobic digestion. Biores Technol 391:129919. https://doi.org/10.1021/acs.est.2c09631
Mahdy A, Bi S, Song Y, Qiao W, Dong R (2020) Overcome inhibition of anaerobic digestion of chicken manure under ammonia-stressed condition by lowering the organic loading rate. Bioresour Tech Reports 9:100359. https://doi.org/10.1016/j.biteb.2019.100359
Massé DI, Rajagopal R, Singh G (2014) Technical and operational feasibility of psychrophilic anaerobic digestion biotechnology for processing ammonia-rich waste. Appl Energy 120:49–55. https://doi.org/10.1016/j.apenergy.2014.01.034
Meng X, Sui Q, Liu J, Yu D, Wang Y, Wei Y (2020) Relieving ammonia inhibition by zero-valent iron (ZVI) dosing to enhance methanogenesis in the high solid anaerobic digestion of swine manure. Waste Manage 118:452–462. https://doi.org/10.1016/j.wasman.2020.08.021
Mlinar S, Weig AR, Freitag R (2022) Influence of NH3 and NH4+ on anaerobic digestion and microbial population structure at increasing total ammonia nitrogen concentrations. Biores Technol 361:127638. https://doi.org/10.1016/j.biortech.2022.127638
Montecchio D, Gallipoli A, Gianico A, Mininni G, Pagliaccia P, Braguglia C (2017) Biomethane potential of food waste: modeling the effects of mild thermal pretreatment and digestion temperature. Environ Technol 38:1452–1464. https://doi.org/10.1080/09593330.2016.1233293
Nasr N, Elbeshbishy E, Hafez H, Nakhla G, Hesham El Naggar M (2012) Comparative assessment of single-stage and two-stage anaerobic digestion for the treatment of thin stillage. Biores Technol 111:122–126. https://doi.org/10.1016/j.biortech.2012.02.019
Ngo T, Khudur LS, Krohn C, Hassan S, Jansriphibul K, Hakeem IG, Shah K, Surapaneni A, Ball AS (2023) Wood biochar enhances methanogenesis in the anaerobic digestion of chicken manure under ammonia inhibition conditions. Heliyon 9:e21100. https://doi.org/10.1016/j.heliyon.2023.e21100
Nie H, Jacobi HF, Strach K, Xu C, Zhou H, Liebetrau J (2015) Mono-fermentation of chicken manure: ammonia inhibition and recirculation of the digestate. Biores Technol 178:238–246. https://doi.org/10.1016/j.biortech.2014.09.029
Niu Q, Qiao W, Qiang H, Hojo T, Li YY (2013) Mesophilic methane fermentation of chicken manure at a wide range of ammonia concentration: stability, inhibition and recovery. Bioresou Tech 137:358–367
Niu Q, Qiao W, Qiang H, Hojo T, Li YY (2013a) Mesophilic methane fermentation of chicken manure at a wide range of ammonia concentration: stability, inhibition and recovery. Bioresour Techn 137:358–367
Niu Q, Takemura Y, Kubota K, Li Y-Y (2015b) Comparing mesophilic and thermophilic anaerobic digestion of chicken manure: Microbial community dynamics and process resilience. Waste Manage 43:114–122. https://doi.org/10.1016/j.wasman.2016.10.038
O’Connor S, Ehimen E, Pillai SC, Black A, Tormey D, Bartlett J (2021) Biogas production from small-scale anaerobic digestion plants on European farms. Renew Sustain Energy Rev 139:110580. https://doi.org/10.1016/j.rser.2020.110580
O’Connor J, Mickan BS, Gurung SK, Siddique KHM, Leopold M, Bolan NS (2023) Enhancing nutrient recovery from food waste anaerobic digestate. Biores Technol 390:129869. https://doi.org/10.1016/j.biortech.2023.129869
Pan J, Chen X, Sheng K, Yu Y, Zhang C, Ying Y (2013) Effect of ammonia on biohydrogen production from food waste via anaerobic fermentation. Int j Hydrog Energy 38(29):12747–12754
Paranjpe A, Saxena S, Jain P (2023) A review on performance improvement of anaerobic digestion using co-digestion of food waste and sewage sludge. J Environ Manage 338:117733. https://doi.org/10.1016/j.jenvman.2023.117733
Park J-H, Yoon J-J, Kumar G, Jin Y-S, Kim S-H (2018) Effects of acclimation and pH on ammonia inhibition for mesophilic methanogenic microflora. Waste Manage 80:218–223. https://doi.org/10.1016/j.wasman.2018.09.016
Pedizzi C, Lema JM, Carballa M (2017) Enhancing thermophilic co-digestion of nitrogen-rich substrates by air side-stream stripping. Biores Technol 241:397–405. https://doi.org/10.1016/j.biortech.2017.05.113
Peng X, Zhang S, Li L, Zhao X, Ma Y, Shi D (2018) Long-term high-solids anaerobic digestion of food waste: effects of ammonia on process performance and microbial community. Biores Technol 262:148–158. https://doi.org/10.1016/j.biortech.2018.04.076
Peng Y, Li L, Yuan W, Wu D, Yang P, Peng X (2022) Long-term evaluation of the anaerobic co-digestion of food waste and landfill leachate to alleviate ammonia inhibition. Energy Convers Manage 270:116195. https://doi.org/10.1016/j.enconman.2022.116195
Peng L, Li Y, Li Q, Liang C, Nasr M, Xu Y, Liu Y, Zhou Y (2023a) The effect of free ammonia on ammonium removal and N2O production in a consortium of microalgae and partial nitritation cultures. Chem Eng J 474:145572. https://doi.org/10.1016/j.cej.2023.145572
Peng Y, Li L, Dong Q, Yang P, Liu H, Ye W, Wu D, Peng X (2023b) Evaluation of digestate-derived biochar to alleviate ammonia inhibition during long-term anaerobic digestion of food waste. Chemosphere 311:137150. https://doi.org/10.1016/j.chemosphere.2022.137150
Peng Y, Li L, Yang P, Liu H, Ye W, Xue Z, Peng X, Wang X (2023c) Integrated genome-centric metagenomic and metaproteomic analyses unravel the responses of the microbial community to ammonia stress. Water Res 242:120239. https://doi.org/10.1016/j.watres.2023.120239
Poirier S, Desmond-Le Quéméner E, Madigou C, Bouchez T, Chapleur O (2016) Anaerobic digestion of biowaste under extreme ammonia concentration: identification of key microbial phylotypes. Biores Technol 207:92–101. https://doi.org/10.1016/j.biortech.2016.01.124
Poirier S, Madigou C, Bouchez T, Chapleur O (2017) Improving anaerobic digestion with support media: mitigation of ammonia inhibition and effect on microbial communities. Biores Technol 235:229–239. https://doi.org/10.1016/j.biortech.2017.03.099
Pongsopon M, Woraruthai T, Anuwan P, Amawatjana T, Tirapanampai C, Prombun P, Kusonmano K, Weeranoppanant N, Chaiyen P, Wongnate T (2023) Anaerobic co-digestion of yard waste, food waste, and pig slurry in a batchexperiment: an investigation on methane potential, performance, and microbialcommunity. Bioresour Technol Rep 21:101364. https://doi.org/10.1016/j.biteb.2023.101364
Procházka J, Dolejš P, Máca J, Dohányos M (2012a) Stability and inhibition of anaerobic processes caused by insufficiency or excess of ammonia nitrogen. Appl Microbio Biotech. 93:439–447
Procházka J, Dolejš P, Máca J, Dohányos M (2012b) Stability and inhibition of anaerobic processes caused by insufficiency or excess of ammonia nitrogen. Appl Microbio Biotech 93:439–447
Provolo G, Perazzolo F, Mattachini G, Finzi A, Naldi E, Riva E (2017) Nitrogen removal from digested slurries using a simplified ammonia stripping technique. Waste Manage 69:154–161. https://doi.org/10.1016/j.wasman.2017.07.047
Qian W, Peng Y, Li X, Zhang Q, Ma B (2017) The inhibitory effects of free ammonia on ammonia oxidizing bacteria and nitrite oxidizing bacteria under anaerobic condition. Biores Technol 243:1247–1250. https://doi.org/10.1016/j.wasman.2017.07.047
Rajagopal R, Massé DI, Singh G (2013) A critical review on inhibition of anaerobic digestion process by excess ammonia. Biores Tech 143:632–641
Ren Y, Yu M, Wu C, Wang Q, Gao M, Huang Q, Liu Y (2018) A comprehensive review on food waste anaerobic digestion: Research updates and tendencies. Biores Technol 247:1069–1076. https://doi.org/10.1016/j.biortech.2017.09.109
Rivera F, Akpan J, Prádanos P, Hernández A, Palacio L, Muñoz R (2023) Side-stream membrane-based NH3 extraction to improve the anaerobic digestion of poultry manure. J Water Process Eng 54:103990. https://doi.org/10.1016/j.jwpe.2023.103990
Rosas-Mendoza ES, Alvarado-Vallejo A, Vallejo-Cantú NA, Velasco-Santos C, Alvarado-Lassman A (2024) Valorization of the complex organic waste in municipal solid wastes through the combination of hydrothermal carbonization and anaerobic digestion. Renew Energy 231:120916
Ruiz-Sánchez J, Campanaro S, Guivernau M, Fernández B, Prenafeta-Boldú F (2018) Effect of ammonia on the active microbiome and metagenome from stable full-scale digesters. Biores Technol 250:513–522. https://doi.org/10.1016/j.biortech.2017.11.068
Sailer G, Eichermüller J, Poetsch J, Paczkowski S, Pelz S, Oechsner H, Müller J (2021) Characterization of the separately collected organic fraction of municipal solidwaste (OFMSW) from rural and urban districts for a one-year period in Germany. Waste Manag 131:471–482. https://doi.org/10.1016/j.wasman.2021.07.004
Sanjaya EH, Cheng H, Li Y-Y (2020) Mesophilic methane fermentation performance and ammonia inhibition of fish processing wastewater treatment using a self-agitated anaerobic baffled reactor. Biores Technol 313:123644. https://doi.org/10.1016/j.biortech.2020.123644
Shen F, Yuan H, Pang Y, Chen S, Zhu B, Zou D, Liu Y, Ma J, Yu L, Li X (2013) Performances of anaerobic co-digestion of fruit & vegetable waste (FVW) and food waste (FW): single-phase versus two-phase. Biores Technol 144:80–85. https://doi.org/10.1016/j.biortech.2013.06.099
Shi X, Lin J, Zuo J, Li P, Li X, Guo X (2017) Effects of free ammonia on volatile fatty acid accumulation and process performance in the anaerobic digestion of two typical bio-wastes. J Environ Sci 55:49–57. https://doi.org/10.1016/j.jes.2016.07.006
Shi X, Zuo J, Zhang M, Wang Y, Yu H, Li B (2019) Enhanced biogas production and in situ ammonia recovery from food waste using a gas-membrane absorption anaerobic reactor. Biores Technol 292:121864. https://doi.org/10.1016/j.biortech.2019.121864
Sung S, Liu T (2003) Ammonia inhibition on thermophilic anaerobic digestion. Chemosphere 53:43–52. https://doi.org/10.1016/S0045-6535(03)00434-X
Tian H, Fotidis IA, Mancini E, Angelidaki I (2017) Different cultivation methods to acclimatise ammonia-tolerant methanogenic consortia. Biores Technol 232:1–9. https://doi.org/10.1016/S0045-6535(03)00434-X
Tian H, Fotidis IA, Mancini E, Treu L, Mahdy A, Ballesteros M, González-Fernández C, Angelidaki I (2018a) Acclimation to extremely high ammonia levels in continuous biomethanation process and the associated microbial community dynamics. Biores Technol 247:616–623. https://doi.org/10.1016/j.biortech.2017.09.148
Tian H, Karachalios P, Angelidaki I, Fotidis IA (2018b) A proposed mechanism for the ammonia-LCFA synergetic co-inhibition effect on anaerobic digestion process. Chem Eng J 349:574–580. https://doi.org/10.1016/j.cej.2018.05.083
Tian H, Mancini E, Treu L, Angelidaki I, Fotidis IA (2019) Bioaugmentation strategy for overcoming ammonia inhibition during biomethanation of a protein-rich substrate. Chemosphere 231:415–422. https://doi.org/10.1016/j.chemosphere.2019.05.140
Tiwari BR, Brar SK, Surampalli RY (2023) Enhancing thermophilic anaerobic digestion of municipal sludge: an investigation. J Water Process Engineering 56:104293. https://doi.org/10.1016/j.jwpe.2023.104293
Wang H, Fotidis IA, Angelidaki I (2015) Ammonia effect on hydrogenotrophic methanogens and syntrophic acetate-oxidizing bacteria. FEMS Microbiol Ecology. https://doi.org/10.1093/femsec/fiv130
Wang Z, Wang S, Hu Y, Du B, Meng J, Wu G, Liu H, Zhan X (2022) Distinguishing responses of acetoclastic and hydrogenotrophic methanogens to ammonia stress in mesophilic mixed cultures. Water Res 224:119029. https://doi.org/10.1016/j.watres.2022.119029
Wang S, Wang Z, Usman M, Zheng Z, Zhao X, Meng X, Hu K, Shen X, Wang X, Cai Y (2023) Two microbial consortia obtained through purposive acclimatization as biological additives to relieve ammonia inhibition in anaerobic digestion. Water Res 230:119583. https://doi.org/10.1016/j.watres.2023.119583
Westerholm M, Lev´en, L., Schnürer, A. (2012) Bioaugmentation of syntrophic acetate-oxidizing culture in biogas reactors exposed to increasing levels of ammonia. Appl Environ Microb 78(21):7619–7625. https://doi.org/10.1128/AEM.01637-12
Wu D, Li L, Zhao X, Peng Y, Yang P, Peng X (2019) Anaerobic digestion: A review on process monitoring. Renew Sustain Energy Rev 103:1–12
Xiao Y, Yang H, Zheng D, Liu Y, Deng L (2022a) Alleviation of ammonia inhibition in dry anaerobic digestion of swine manure. Energy 253:124149. https://doi.org/10.1016/j.energy.2022.124149
Xiao Y, Yang H, Zheng D, Liu Y, Deng L (2022b) Alleviation of ammonia inhibition in dry anaerobic digestion of swine manure. Energy 253:124149
Xu J, Khanal SK, Kang Y, Zhu J, Huang X, Zong Y, Xie L (2022) Role of interspecies electron transfer stimulation in enhancing anaerobic digestion under ammonia stress: Mechanisms, advances, and perspectives. Biores Tech 360:127558
Yan M, Fotidis IA, Tian H, Khoshnevisan B, Treu L, Tsapekos P, Angelidaki I (2019) Acclimatization contributes to stable anaerobic digestion of organic fraction of municipal solid waste under extreme ammonia levels: Focusing on microbial community dynamics. Biores Technol 286:121376. https://doi.org/10.1016/j.biortech.2019.121376
Yan M, Treu L, Campanaro S, Tian H, Zhu X, Khoshnevisan B, Tsapekos P, Angelidaki I, Fotidis IA (2020) Effect of ammonia on anaerobic digestion of municipal solid waste: inhibitory performance, bioaugmentation and microbiome functional reconstruction. Chem Eng J 401:126159. https://doi.org/10.1016/j.cej.2020.126159
Yang Z, Wang W, He Y, Zhang R, Liu G (2018) Effect of ammonia on methane production, methanogenesis pathway, microbial community and reactor performance under mesophilic and thermophilic conditions. Renewable Energy 125:915–925. https://doi.org/10.1016/j.renene.2018.03.032
Yang Z, Wang W, Liu C, Zhang R, Liu G (2019) Mitigation of ammonia inhibition through bioaugmentation with different microorganisms during anaerobic digestion: selection of strains and reactor performance evaluation. Water Res 155:214–224. https://doi.org/10.1016/j.watres.2019.02.048
Yang Z, Sun H, Zhao Q, Kubonova M, Zhang R, Liu G, Wang W (2020) Long-termevaluation of bioaugmentation to alleviate ammonia inhibition during anaerobicdigestion: process monitoring, microbial community response, and methanogenic pathway modeling. Chem Eng J 399:125765. https://doi.org/10.1016/j.cej.2020.125765
Yang Z, Sun H, Kurbonova M, Zhou L, Arhin SG, Papadakis VG, Goula MA, Liu G, Zhang Y, Wang W (2022) Simultaneous supplementation of magnetite and polyurethane foam carrier can reach a Pareto-optimal point to alleviate ammonia inhibition during anaerobic digestion. Renew Energy 189:104–116. https://doi.org/10.1016/j.renene.2022.02.092
Ye M, Zhu A, Sun B, Qin Y, Li Y-Y (2022) Methanogenic treatment of dairy product wastewater by thermophilic anaerobic membrane bioreactor: ammonia inhibition and microbial community. Biores Technol 357:127349. https://doi.org/10.1016/j.biortech.2022.127349
Ye X, Jia Z, Liu Y, Wang C, Cao C, Zhang Y, Han T, Wang L, Guo T, Xi Y (2024) Constructing carbon-based materials loaded with MOFs to realize efficient anaerobic digestion of rural organic waste. Fuel 355:129536. https://doi.org/10.1016/j.fuel.2023.129536
Yenigün O, Demirel B (2013) Ammonia inhibition in anaerobic digestion: a review. Process Biochem 48:901–911. https://doi.org/10.1016/j.procbio.2013.04.012
Yu D, Wang T, Liang Y, Liu J, Zheng J, Chen M, Wei Y (2021) Delivery and effects of proton pump inhibitor on anaerobic digestion of food and kitchen waste under ammonia stress. J Hazard Mater 416:126211. https://doi.org/10.1016/j.jhazmat.2021.126211
Zhang W, Xing W, Li R (2018) Real-time recovery strategies for volatile fatty acid-inhibited anaerobic digestion of food waste for methane production. Biores Technol 265:82–92. https://doi.org/10.1016/j.biortech.2018.05.098
Zhang H, Yuan W, Dong Q, Wu D, Yang P, Peng Y, Li L, Peng X (2022) Integrated multi-omics analyses reveal the key microbial phylotypes affecting anaerobic digestion performance under ammonia stress. Water Res 213:118152. https://doi.org/10.1016/j.watres.2022.118152
Zhang H, Fu Z, Guan D, Zhao J, Wang Y, Zhang Q, Xie J, Sun Y, Guo L, Wang D (2023) A comprehensive review on food waste anaerobic co-digestion: current situation and research prospect. Process Saf Environ Prot 179(2023):546–558. https://doi.org/10.1016/j.psep.2023.09.030
Zhao P, Wang Y, Lin Z, Zhou J, Chai H, He Q, Li Y, Wang J (2019) The alleviative effect of exogenous phytohormones on the growth, physiology and gene expression of tetraselmis cordiformis under high ammonia-nitrogen stress. Biores Technol 282:339–347. https://doi.org/10.1016/j.biortech.2019.03.031
Zhuang L, Ma J, Yu Z, Wang Y, Tang J (2018) Magnetite accelerates syntrophic acetate oxidation in methanogenic systems with high ammonia concentrations. Microb Biotechnol 11:710–720. https://doi.org/10.1111/1751-7915.13286
Zhuo Y, Han Y, Qu Q, Cao Y, Peng D, Li Y (2018) Pre-separation of ammonium content during high solid thermal-alkaline pretreatment to mitigate ammonia inhibition: kinetics and feasibility analysis. Water Res 139:363–371. https://doi.org/10.1016/j.watres.2018.03.064
Ziganshin AM, Liebetrau J, Pröter J, Kleinsteuber S (2013) Microbial community structure and dynamics during anaerobic digestion of various agricultural waste materials. Appl Microbio Biotech 97:5161–5174
Acknowledgements
This work was supported by the Gansu Province University Industry Support Program Project (2020c-38); Gansu Province Key R&D Project Program (2021-0201-GXC-0145).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Editorial responsibility: S.Mirkia.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, J., Zhang, J., Du, X. et al. Ammonia inhibition in anaerobic digestion of organic waste: a review. Int. J. Environ. Sci. Technol. (2024). https://doi.org/10.1007/s13762-024-06029-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13762-024-06029-1