Abstract
The Mediterranean Basin economies of Spain, Italy, Greece, and Turkey depend on the table olive and olive oil industries. Table olive processing wastewater characteristics and quantity depend on olive varietal and processing methods. Olives and processing methods provide phenol, suspended particles, dissolved inorganic solids, and refractory organics. Due of its complexity, conventional methods struggle to tackle table olive processing wastewater. A novel approach to enhanced oxidation processes integrates many ways to boost hydroxyl radical formation and scale up efficiency. UV assisted sono-Fenton process as a modified Fenton process was applied to table olive washing wastewater to achieve high formation of hydroxyl radicals. When UV assisted sono-Fenton experiments executed to determine the optimum reaction conditions, the effects of hydrogen peroxide, ferrous ion concentrations and reaction time on the oxidation of table olive washing wastewater investigated by using a statistical experimental design. Chemical oxygen demand (COD), total organic carbon (TOC) and phenol removal efficiencies were examined by keeping the pH constant. The UV assisted sono-Fenton process achieved 53% phenol, 68% TOC, and 80% COD removal efficiencies. The results show that the UV assisted sono-Fenton process can treat effectively table olive processing wastewater. Optimum reaction conditions for the UV assisted sono-Fenton process were determined. UV assisted sono-Fenton process provides a significant reduction in reaction time and minimizes the costs of process associated with low chemical requirements. So, these optimum reaction conditions were resulted in low sludge production at the UV assisted sono-Fenton process while treating table olive processing wastewater.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
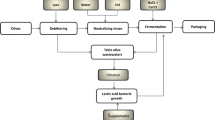
The table olive and olive oil industries are significant agro-industrial sectors that have a vital impact on the economies of Mediterranean Basin countries, such as Spain, Italy, Greece, and Turkey. Moreover, there has been a significant increase in the production of table olives in recent years, particularly in Mediterranean countries (Aldana et al. 2021). Table olives can be categorized into three primary classifications: green, black, and blackened, as a result of oxidation during the production process. The primary commercially available types of table olives consist of Spanish-style green olives, Californian-style black ripe olives, and naturally black olives preserved in brine. The primary method employed for the production of table olives is referred to as "Spanish-style" processing, which constitutes around 50% of the total output (Delgado et al. 2022). The characteristics and quantity of wastewater generated during the processing of table olives depend on the type of olive and the methods used for processing. Phenol, suspended particles, dissolved inorganic solids, and refractory organics are some of the pollutants included in table olive washing wastewater (TOPW), which is obtained from olives and processing methods. The presence of these chemical compounds is harmful to living organisms and leads to a decrease in the nutritional quality of olives (Fernandez et al. 1997). The production of California-style black ripe olives has the highest capacity for creating pollutants, with a maximum total volume of around 6 L per kilogram of olives produced. Spanish-style green olive processing generates two types of wastewater: alkaline wastewater from debittering and washing processes, and acidic wastewater from fermentation brine processes. However, these wastewaters were typically released into the environment untreated, which contributed to water pollution. Areas in which the effluent was released were also impacted, resulting in unpleasant odors and the coloring of natural waters. Furthermore, the presence of hazardous substances in the soil and aquifers has emerged as a substantial issue following unregulated discharge or inadequate treatment.
Table olive washing wastewater (TOPW) can be treated using conventional treatment processes. However, some conventional treatment process resulted in low removal efficiency, while other conventional treatment processes had high costs despite providing limited treatment. The complex characterization of the wastewater makes it difficult to easily accomplish the treatment of TOPW using conventional treatment processes. The primary concern in evaluating treatment processes for wastewater is the seasonal variation in wastewater composition, particularly the high levels of organic load, phenol, and salts.
Advanced oxidation processes (AOPs) are a good way to treat or break down organic pollutants by hydroxyl radicals that are non-selective and reacting with refractory organic compounds. The most effective advanced oxidative processes (AOPs) for degrading organic pollutants in wastewater is Fenton process. The Fenton process occurred by the reactions from hydrogen peroxide and iron salts hydrogen peroxide produces ·OH radicals.
The Fenton process effectively applied to treat various types of industrial effluents, as a single-, pre- and/or post-treatment methods such as leachate wastewater, pharmaceutical wastewater etc. (Lyngsie et al. 2018; Deng et al. 2022).
Ultrasounds are recognized as a subsequent form of enhanced oxidation. The ultrasound (US) process involves the occurrence of cavitation, which includes the formation, growth, and collapse of bubbles in a liquid in an implosive manner (Bilińska and Gmurek 2021). When bubbles collapse under high pressure, the collapse generates "hot spots" where water dissociation and the creation of radical oxygen species (ROS), such as hydroxyl radicals, occur. This is a crucial step in the ultrasound process called the advanced oxidation process.
where -))) denotes to the ultrasound waves.
A novel strategy in advanced oxidation processes involves the integration of various techniques to enhance the generation of hydroxyl radicals and improve the treatment of a wide range of compounds on a larger scale. Researchers have conducted multiple studies to enhance the generation of hydroxyl radicals using improved oxidation methods (Nidheesh et al. 2023). The utilization of ultrasound in the combined treatment procedure exceeds its usage in a single process (Basturk and Karatas 2014; Gogate and Pandit 2004). The ultrasound process (US) has been integrated with other AOPs, including UV light (Zhong et al. 2011; Kim et al. 2007; Bagal and Gogate 2014), TiO2 photocatalysis (Zarei et al. 2010), and ozone (Abu Amr and Aziz 2012).
The integration of ultrasonic with the Fenton process has proven to be effective in treating wastewater by eliminating a wide range of pollutants. Furthermore, the utilization of ultrasonic sound enhances the efficiency of the Fenton process by promoting the rapid generation of hydroxyl radicals. Ultrasonic irradiation reduces the requirement for chemical reagents and overcomes the limitations of the reactions in the Fenton process (Adityosulindro et al. 2017; Babuponnusami and Muthukumar 2011). In general, the cost of hybrid ultrasound processes, such as sono-Fenton and sono-photo-Fenton, is lower compared to other hybrid or combined advanced oxidation processes, such as ozonation, peroxone (O3/H2O2) process, and UV/H2O2 process (Mahamuni and Adewuyi 2010).
Further researches on enhancement of Fenton process and hybrid Fenton process is needed to show the economic and commercial feasibility of these processes via optimization of reaction conditions and limitations of sludge production. For this aim, UV assisted sono-Fenton process called as combined process is applied to table olive processing wastewater. The combined treatment process of Fenton reaction, ultrasound and ultraviolet light is employed to evaluate the synergistic effect of Fenton reactions, sonolysis and photolysis on TOPW treatment. The UV assisted sono-Fenton process needs fewer chemicals than the classical Fenton process. UV assisted sono-Fenton process can achieve small amount of sludge production because of low amount of catalyst requirement. In this study, modified Fenton process with ultrasound process is assisted with ultraviolet light in order to accelerate hydroxyl radical formation, to minimize the need for additional chemicals, to minimize and optimize reaction time and to formation ferrous ion to ferric ion, to promote the Fe+2/Fe+3 redox cycle etc. UV assisted sono-Fenton process is the alternative method to minimize drawbacks of classical Fenton process, this is the aim of this research. To achieve this aim, Box-Behnken statically design is also applied to optimization of reaction conditions of UV assisted sono-Fenton process.
Materials and methods
The table olive processing wastewater was obtained from a factory at Manisa in Turkey at 2020. At this table olive processing industry, the type of table olives is consisted of naturally black olives preserved in brine. Table 1 shows the characteristics of the wastewater from table olive processing used in the study. The characterization of table olive processing effluent poses challenges in treatment due to its high pollutant contents. The wastewater generated by processing table olives contains significant quantities of organic matter and phenolic compounds. The concentration of suspended solids is likewise relatively high. Furthermore, wastewater has a deep brown color. The analysis methods for the parameters are shown in Table 1 (APHA 2017).
The UV assisted sono-Fenton process used to conduct experimental research. The first step in the UV assisted Sono-Fenton process is the addition of wastewater into a US reactor with a volume of 1500 mL. Following that, the ferrous ion was added and used as a catalyst, and hydrogen peroxide was added as an oxidant into the US reactor to start the sono-Fenton Process. The oxidant dosages were determined within the range of 2600–7600 mg/L using the theoretical hydrogen peroxide demand, which was dependent on the initial chemical oxygen demand of the wastewater. In addition, catalyst doses were selected within the range of 250–500 mg/L, which were determined to be the optimal catalyst/oxidant molar ratio. During sono-Fenton process, the intensity of the ultrasound process is set at 100 W/cm2, and the reaction time of ultrasound process is varied between 5 and 30 min. Following the sono-Fenton process, the treated wastewater was pumped into the UV reactor, and the UV reactor was started. Ultraviolet radiation was applied to effluent wastewater for pre-determined reaction time. The reaction time of the UV reactor was adjusted within a range of 5 to 30 min. The pH value was kept at its raw pH value during the experiments to eliminate the addition of chemicals. The effects of varying Fe+2 (250–500 mg/L), H2O2 (2600–7600 mg/L), and reaction time (5–30 min) on chemical oxygen demand (COD), phenol, and total organic carbon (TOC) were analyzed. A power generator, ultrasonic transducer, and glass reactor manufactured under the MEINHARDT trademark were used in ultrasound studies. In addition, the pump was used inside the system to allow the circulation of wastewater back into the process. The ultrasonic transducer has a height and diameter of 75 mm, and it weighs around 1000 g. The converter has the capability to function at a frequency of 850 kHz. The MEINHARDT UST02 model glass reactor was used in the ultrasonic (US) experimental setup. The double-walled glass reactor has a 1500-mL capacity and a 500-mm diameter. Figure 1a depicts the glass reactor that was used. The power supply intensity was adjusted to 100 W/cm2. Figure 1a also shows the ultrasound power generator that was utilized.
PURFECT-6 model stainless steel surface UV reactor which includes 24 W UV lamp was used as the second reactor of the experimental studies. The UV reactor is shown in Fig. 1b. The study evaluated the effect of reaction time of US and UV processes, ferrous ion concentration, and hydrogen peroxide concentration on the UV assisted sono-Fenton process. The Box-Behnken design approach was employed as an efficient and valuable method for optimizing three variable response functions. A variation of the central composite experimental design, known as the Box-Behnken design (BBD), stands out as an independent, rotatable quadratic design devoid of embedded factorial or fractional factorial designs (Ragonese et al. 2002). The Box-Behnken design, among other statistical experimental design methods, requires fewer runs compared to others, such as only 15 runs for a 3-factor experiment design. Additionally, this method enables the calculation of response functions at intermediate levels that may not be experimentally studied (Sastry and Khan 1998; Hamed and Sakr 2001; Ferreira et al. 2007). Highlighted as an effective approach, the Box-Behnken design optimizes three-variable response functions, predicting the responses of fitted models through ANOVA tests (Charles and Kennneth 1999; Abbasi et al. 1987). In this study, Design Expert 10.0 was used to design experiments and evaluate the results. The Box-Behnken experimental design was used to determine the independent variables of US/UV reaction time, ferrous ion concentration, and hydrogen peroxide concentration. The variables include the US or UV reaction time denoted as "X1", ferrous ion concentration represented by "X2", and hydrogen peroxide concentration defined as "X3". The range of independent variables for the UV assisted sono-Fenton process is presented in Table 2. The variables are categorized into three levels: low, middle, and high. These levels are presented by the values −1, 0, and + 1 correspondingly, as seen in Table 2. The dependent variables are defined as the efficiencies of TOC removal (Y1), COD removal (Y2), and Phenol removal (Y3).
Table 3 shows the experimental conditions of the Box-Behnken experiment design that executed to determine the effect of independent variables on the UV assisted sono-Fenton process.
Results and discussion
The objective of this study is to assess the efficiency of the UV assisted sono-Fenton process. Accordingly, various dependent variables, including reaction time for the US/UV process, the concentrations of ferrous ions and hydrogen peroxide, were examined to evaluate the efficiency of these processes.
The Box-Behnken statistical design method was employed to conduct the experiments. This approach was chosen for its effectiveness and utility in optimizing three-variable response functions, with the response of the fitted model predicted through ANOVA tests (Çokay 2018). The Box-Behnken Design was utilized to evaluate the effects of independent variables on dependent variables in the UV assisted sono-Fenton process. Detailed information on the experimental conditions for each run and the results of the Box-Behnken Experimental Design are provided in Table 4. Additionally, Table 5 presents both observed and predicted results, expressed as removal percentages using the Box-Behnken design.
The regression model
The application of RSM offers an empirical relationship between the response function and the independent variables. The mathematical relationship between the response function (Y) and the independent variables (X) can be approximated by a quadratic (second order) polynomial equation as follows:
This approach was selected as relatively fewer combinations of the variables were chosen to estimate a potential complex response function. A total of 15 experiments are needed to calculate 9 coefficients of the second-order polynomial regression model. This model contains one block term, three linear terms, three quadratic terms, and three interaction terms. The coefficients of the response functions for different dependent variables were determined correlating the experimental results with the relevant functions used in a Stat-Ease regression program (Catalkaya and Kargi 2009). Equations present different response functions with the determined coefficients Eq. (6) to (8).
-
The response function for percent TOC removal (Y1) has the following forms:
$$\begin{aligned} {\text{Y}}_{{1}} & = {221}.{94} + {1}.{{{92929}}{\text{X}}}_{{1}} - 0.{{\mathrm{74}}\text{X}}_{{2}} - 0.0{\text{3X}}_{{3}} + 0.0014{\text{X}}_{{1}} {{\mathrm{14}}{\text{X}}}_{{2}} - 0.0003{\text{X}}_{{1}} {\text{X}}_{{3}} \\ & \quad + 0.000017{\text{X}}_{{2}} {\text{X}}_{{3}} - 0.037{\text{X}}_{{1}}^{{2}} + 0.0008{\text{X}}_{{2}}^{{2}} + {2}.{{9}}{\text{E}}^{{ - 0{6}}} {\text{X}}_{{3}}^{{2}} \quad \quad {\text{R}}^{{2}} = 0.{98} \\ \end{aligned}$$(6) -
The response function for percent COD removal (Y2) has the following forms:
$$\begin{aligned} {\text{Y}}_{{2}} & = {128}.{66} + {3}.{414}{\text{X}}_{{1}} - 0.66{\text{X}}_{{2}} - 0.0084{\text{X}}_{{3}} + 0.0042{\text{X}}_{{1}} {\text{X}}_{{2}} - 0.0005{\text{X}}_{{1}} {\text{X}}_{{3}} \\ & \quad - {8}*{\text{E}}^{{ - 0{6}}} {\text{X}}_{{2}} {\text{X}}_{{3}} - 0.031{\text{X}}_{{1}}^{{2}} + 0.000{\text{8X}}_{{2}}^{{2}} + {2}.{5}*{\text{E}} - 06{\text{ X}}_{{3}}^{{2}} \quad \quad {\text{R}}^{{2}} = 0.{98} \\ \end{aligned}$$(7) -
The response function for percent Phenol removal (Y3) has the following forms:
$$\begin{aligned} {\text{Y}}_{{3}} & = 102.68 - 3.84{\text{X}}_{1} - 0.17{\text{X}}_{{2}} + 0.004 {\text{X}}_{3} + 0.01{\text{X}}_{{1}} {\text{X}}_{{2}} - 0.0001{\text{X}}_{1} {\text{X}}_{{3}} \\ & \quad + {5.352}*{\text{E}}^{{-06}} {\text{X}}_{{2}} {\text{X}}_{{3}} - 0.021{\text{X}}_{1}^{{2}} - 0,00013{\text{X}}_{{2}}^{{2}} - {5.5}*{\text{E}}^{ - 07} *{\text{X}}_{{3}}^{{2}} & \quad {\text{R}}^{{2}} = 0.99 \\ \end{aligned}$$(8)
The predictability of the model is at 95% confidence according to interval analysis of variance (ANOVA). Response function predictions are in good agreement with the experimental data with a coefficient of determination (R2) of larger than 0.99. Furthermore, the computed F value is much greater than that of the tabular F0.01 (14, 14) value of 3.70 suggesting that the treatment is highly significant. P values of less than 0.05 for any factor in analysis of variance (ANOVA) indicated a significant effect of the corresponding variable on the response.
Chemical oxygen demand removal
The achieved chemical oxygen demand (COD) removal efficiencies through the UV assisted sono-Fenton process, under varying concentrations of hydrogen peroxide and a constant intensity of 100 W/m2, are depicted in Fig. 2.
In the context of the sono-Fenton/UV combined oxidation processes, the COD removal efficiency reached 85% at an average hydrogen peroxide concentration of 5100 mg/L. This result was established under experimental conditions involving a reaction time of 30 min with ultrasound and ultraviolet, along with a ferrous ion concentration of 450 mg/L. According to the minimum, average and maximum hydrogen peroxide concentrations obtained in the experimental results, the optimum COD removal efficiency was determined with a hydrogen peroxide dose exceeding 5000 mg/L. Notably, increasing hydrogen peroxide dose showed a positive effect on COD removal efficiency. The oxidant dose emerged as a main factor influencing removal efficiencies. At specific reaction conditions involving a ferrous dose of 250 mg/L and a hydrogen peroxide dose of 7500 mg/L, a COD removal efficiency of 80% was achieved within a 5-min reaction time. A higher hydrogen peroxide than 7000 mg/L dose is recommended for those wishing to optimize or minimize reaction times and minimize catalyst dose which increase the sludge volume.
Beltran-Heradia et al.(1999) observed that utilizing ozone alone in the treatment of table olive processing wastewater (TOPW) achieves a COD removal efficiency ranging from 42 to 55%. However, it is insufficient for effectively removing organic compounds using only ozone process, table olive wastewater was treated using the ozone/H2O2/UV process, yielding approximately 80% COD removal efficiency (Chatzisymeon et al. 2008). However, this advanced oxidation process requires the use of more chemicals. This leads to higher operating costs and higher sludge production. In contrast, UV assisted sono-Fenton Process, a COD removal efficiency of 80% was achieved within a 5-min reaction time at optimum reaction conditions involving a ferrous dose of 250 mg/L and a hydrogen peroxide dose of 7500 mg/L.
It is underscored that the reaction time in the ultrasound process holds significance for attaining maximum COD removal efficiency. Additionally, hydrogen peroxide concentration proves to be a crucial parameter influencing COD removal efficiency. The model F-value, standing at 2195, attests to the significance of the model. Noteworthy model terms include X1, X3, X1X2, X1X3, X22, and X32. However, the adjustment of ferrous ion concentration did not yield a significant change in COD removal efficiency across various hydrogen peroxide concentrations and reaction times.
Total organic carbon removal
The total organic carbon (TOC) removal efficiencies achieved through the UV assisted sono-Fenton process under varying concentrations of hydrogen peroxide and a constant intensity of 100 W/m2 are shown in Fig. 3. When the hydrogen peroxide dose was set to its maximum value during ultrasound and ultraviolet reaction times (5 min.) with a ferrous concentration of 250 mg/L, the TOC removal efficiency was observed to be approximately 56%.
Analysis of the results revealed that TOC removal efficiency demonstrated an increasing trend corresponding to higher hydrogen peroxide concentrations. Specifically, at a reaction time of 10 min and a Fe2+ concentration of 450 mg/L, the TOC removal efficiency reached 60% with the addition of the maximum hydrogen peroxide dose. Consequently, it was observed that increasing hydrogen peroxide concentration during the minimum reaction time positively influenced TOC removal efficiency, while an increase in Fe2+ concentration had a contrary negative effect on the removal efficiency. According to a study conducted by Beltran-Heredia et al. (2000), an approximate 7% total carbon (TC) removal efficiency was achieved after using 10 g of ozone for a duration of five hours. Subsequently, when hydrogen peroxide was added as an oxidant to the ozone system, the TC removal efficiency only increased to 30%, utilizing approximately 340 mg/L of H2O2 and 4.3 g/L of ozone dose. In contrast, UV assisted sono-Fenton Process, a TOC removal efficiency of 53% was achieved within a 5-min reaction time at optimum reaction conditions involving a ferrous dose of 250 mg/L and a hydrogen peroxide dose of 7500 mg/L in this study. UV assisted sono-Fenton process was operated with short reaction times and raw pH values resulted in a minimum operation cost.
Further investigation disclosed that increase in ferrous ion concentration did not significantly enhance TOC removal efficiency. Although an increase in ultrasound or ultraviolet reaction time exerted a modest impact on yield, this increment made a positive contribution to TOC removal efficiency. Notably, the maximum TOC removal efficiencies were achieved when hydrogen peroxide concentration reached its peak, surpassing values obtained at other concentrations. Interpretation of the experimental results suggested a beneficial role of hydrogen peroxide in the mineralization of table olive wastewater.
These observations were substantiated by the ANOVA test, with a model F-value of 3025 signifying the model’s significance. In this context, X1, X2, X3, X1X3, X2X3, X12, X22, and X32 emerged as crucial model terms.
Phenol removal
The phenol removal efficiencies achieved through the UV assisted sono-Fenton process, under varying concentrations of hydrogen peroxide and a constant intensity of 100 W/m2, are depicted in Fig. 4. The phenol removal efficiency ranged approximately from 6 to 50% with a ferrous concentration ranging from 500 to 250 mg/L when the hydrogen peroxide dose was set to its maximum value during 5 min of reaction time. In the other study, Sounni et al. 2018, applied to electrocoagulation process to pretreat olive mill wastewater (OMW), resulting in the removal of phenolic compounds by up to 80% within 50 min. Conversely, the application of UV assisted sono-Fenton process with short reaction time (5 min) yielded approximately 50% phenol removal efficiency in this study. These reaction conditions have advantages, because short reaction times and low ferrous ion requirements resulted in low operation cost and low production of sludge. This is the aim of this research.
Examination of the results revealed a decrease in phenol removal efficiency with prolonged reaction times. Specifically, at a reaction time of 5 min and a Fe2+ concentration of 250 mg/L, the phenol removal efficiency was determined to be 50% across all hydrogen peroxide concentrations. Consequently, it was established that an increase in hydrogen peroxide concentration during the minimum reaction time slightly influenced phenol removal efficiency, whereas an increase in Fe2+ concentration had a negative impact on the efficiency.
Furthermore, it was observed that increasing ferrous ion concentration did not significantly enhance phenol removal efficiency. The experimental results suggested a positive contribution of hydrogen peroxide to the mineralization of table olive wastewater. The model F-value of 667 attested to the significance of the model in this context. In this case X1, X2, X1X2, X12, X32 are significant model terms.
Optimization of reaction conditions
The optimum reaction conditions for all parameters are determined and presented at Table 6. UV assisted sono-Fenton Process, a COD removal efficiency of 80% was achieved within a 5-min reaction time at optimum reaction conditions. Under these reaction conditions, the phenol yield was measured at 53.1%, while the total organic carbon (TOC) removal efficiency reached 67.9%. When experiments were executed with optimum reaction conditions, these removal efficiencies were obtained, These conditions include a reaction duration of 5 min for ultrasonic treatment, an intensity of 100 W/cm2, a reaction time of 5 min for UV treatment, a hydrogen peroxide concentration of 7500 mg/L, and a ferrous ion concentration of 250 mg/L. According to these results, the UV assisted sono-Fenton process is a suitable treatment method to treat table olive production wastewater with a short reaction time and low chemical addition.
Although the disadvantage of advanced oxidation processes is stated as electrical energy consumption, considering the optimum reaction conditions obtained in the UV assisted sono-Fenton process, it can be said that the low reaction time will provide energy savings. In addition, the other advantage of the UV assisted sono-Fenton process is the minimum chemical requirement especially catalyst dose, and it can be said that low volume sludge formation will occur when focusing on the optimum iron/catalyst doses obtained in the UV assisted sono-Fenton process. At the UV assisted sono-Fenton process, low amount of sludge volume observed as 50 ml/L. At our previous study, classical Fenton process applied to table olive processing wastewater. According to results, high amounts of catalyst addition resulted in only 60% removal efficiency. At these reaction conditions, 150 ml/L sludge occurred in these reaction conditions. Sludge production of classical Fenton process was three times greater than the UV assisted sono-Fenton process. So, the UV assisted sono-Fenton process is suitable to treat table olive washing process and to decrease sludge production. According to experimental results and literature survey, UV assisted sono-Fenton process can be applied to table washing processing wastewater at the real area as a pretreatment unit. In addition, application of UV assisted sono-Fenton process is suitable and simple than other advanced oxidation process such as wet air oxidation, photo-catalyst oxidation because of low energy consumption and simple operational conditions.
Conclusion
The UV assisted sono-Fenton process, which is called as a hybrid advanced oxidation process, was applied to treat table olive processing wastewater. The Box-Behnken statistical design method was applied for carrying out experiments, which is a very efficient response surface methodology used to optimize reaction conditions in advanced oxidation processes. The independent variables examined in this study were the reaction time for UV and US processes, the concentration of hydrogen peroxide, and the concentration of ferrous ions. The dependent variables, regarded as objective functions, included COD, TOC, and phenol removal efficiencies. The assessment of the UV assisted sono-Fenton process was centered on the parameters of COD, phenol, and TOC. Different reaction conditions had an impact on the effectiveness of the system for each parameter. The highest removal efficiency for COD, TOC, and phenol were attained at 80%, 68%, and 53%, respectively, under the optimum reaction conditions achieved by thorough statistical analysis. These conditions include a reaction time of 5 min for ultrasonic treatment, an intensity of 100 W/cm2, a reaction time of 5 min for UV treatment, a hydrogen peroxide concentration of 7500 mg/L, and a ferrous ion concentration of 250 mg/L.
Based on these results, the UV assisted sono-Fenton process has significant potential for treating table olive processing wastewater. This process offers higher efficiency, shorter reaction time, and lower chemical requirements and expenses. This research provides significant insights into enhanced oxidation methods and their utilization in the treatment of industrial wastewater. The UV assisted sono-Fenton process can be applied to real wastewater treatments unit before biological treatment methods as a pretreatment process in order to reduce organic load, to minimize toxicity and to eliminate negative effects of refractory organic compounds on microorganisms. In this case, UV assisted sono-Fenton processes can be more effective and environmentally friendly method in table olive processing wastewater.
Operation costs such as chemical costs, reactor investment cost, energy consumption that are the limitation factors for the large-scale development of Fenton and enhanced Fenton processes, should be accurately provided in future works. In addition, the treatment of table washing processing wastewater should be executed with other iron sources such as goethite, hematite, magnetite as a green iron sources in future works. So, effect of iron sources can be evaluated with minimum sludge production and maximum removal efficiency.
References
Abbasi GF, Ahmad M, Wasim M (1987) Optimization of concrete mix proportioning using reduced factorial experimental technique. J Am Concr Inst 9:55–63. https://doi.org/10.14359/15162
Abu Amr SS, Aziz HA (2012) New treatment of stabilized leachate by ozone/Fenton in the advanced oxidation process. Waste Manage 32:1693–1698. https://doi.org/10.1016/j.wasman.2012.04.009
Adityosulindro S, Barthe L, González-Labrada K, Haza UJJ, Delmas H, Julcour C (2017) Sonolysis and sono-Fenton oxidation for removal of ibuprofen in (waste) water. Ultrason Sonochem 39:889–896
Aldana JC, Acero JL, Alvarez PM (2021) Membrane filtration, activated sludge and solar photocatalytic technologies for the effective treatment of table olive processing wastewater. J Environ Chem Eng 9:105743. https://doi.org/10.1016/j.jece.2021.105743
APHA (2017) Standard methods for the examination of water and wastewater, 23rd edn. American Public Health Association, Washington DC
Babuponnusami A, Muthukumar K (2011) Degradation of phenol in aqueous solution by fenton, sono-fenton and sono-photo-fenton methods. Clean-Soil Air Water 39:142–147
Bagal MV, Gogate PR (2014) Wastewater treatment using hybrid treatment schemes based on cavitation and Fenton chemistry: a review. Ultrason Sonochem 21:1–14. https://doi.org/10.1016/j.ultsonch.2013.07.009
Basturk E, Karatas M (2014) Advanced oxidation of reactive blue 181 solution: a comparison. Ultrason Sonochem 21:1881–1885. https://doi.org/10.1016/j.ultsonch.2014.03.026
Beltrán JF, Garcia-Araya FJ, Frades J, Alvarez P, Gimeno O (1999) Effects of single and combined ozonation with hydrogen peroxide or UV radiation on the chemical degradation and biodegradability of debittering table olive industrial wastewaters. Water Res 33:723–732. https://doi.org/10.1016/S0043-1354(98)00239-5
Beltran-Heredia J, Torregrosa J, Dominguez JR, Garcia J (2000) Ozonation of black-table-olive industrial wastewaters: effect of an aerobic biological pretreatment. J Chem Technol Biotechnol 75:561–568. https://doi.org/10.1002/1097-4660(200007)75:7%3c561::AID-JCTB254%3e3.0.CO;2-B
Bilińska L, Gmurek M (2021) Novel trends in AOPs for textile wastewater treatment: enhanced dye by-products removal by catalytic and synergistic actions. Water Resour Ind 26:100160. https://doi.org/10.1016/j.wri.2021.100160
Catalkaya EC, Kargi F (2009) Degradation and mineralization of simazine in aqueous solution by ozone/hydrogen peroxide advanced oxidation. J Environ Eng 135:1357–1364. https://doi.org/10.1061/(ASCE)EE.1943-7870.0000112
Charles RH, Kennneth VT (1999) Fundamental concepts in the design of experiments. University Press, Oxford
Chatzisymeon E, Stypas E, Bousios S, Xekoukoulotakis NP, Mantzavinos D (2008) Photocatalytic treatment of black table olive processing wastewater. J Hazard Mater 154:1090–1097. https://doi.org/10.1016/j.jhazmat.2007.11.014
Çokay E (2018) Hydrogen gas production from food wastes by electrohydrolysis using a statical design approach. Int J Hydrogen Energy 43:10555–10561. https://doi.org/10.1016/j.ijhydene.2018.01.079
Delgado A, Chammem N, Issaoui M, Ammar E (2022) bioactive phytochemicals from Olive (Olea Europaea). Processing Byproducts Chapter 10–1:1–31. https://doi.org/10.1007/978-3-030-63961-7_10-1
Deng F, Jiang J, Sirés I (2022) State-of-the-art review and bibliometric analysis on electro-Fenton process. Carbon Lett 33:17–34. https://doi.org/10.1007/s42823-022-00420-z
Fernandez AG, Adams MR, Fernandez-Diez MJ (1997) Table Olives: production and processing. Springer, New York
Ferreira SLC, Bruns RE, Ferreira HS, Matos GD, David JM, Brandão GC, da Silva EGP, Portugal LA, dos Reis PS, Souza AS, dos Santos WNL (2007) Box-Behnken design: an alternative for the optimization of analytical methods. Anal Chim Acta 597:179–186. https://doi.org/10.1016/j.aca.2007.07.011
Gogate PR, Pandit AB (2004) A review of imperative technologies for wastewater treatment II: hybrid methods. Adv Environ Res 8:553–597. https://doi.org/10.1016/S1093-0191(03)00031-5
Hamed E, Sakr A (2001) Application of multiple response optimization technique to extended release formulations design. J Control Releas 73:329–338. https://doi.org/10.1016/s0168-3659(01)00356-x
Kim JK, Martinez F, Metcalfe IS (2007) The beneficial role of use of ultrasound in heterogeneous Fenton-like system over supported copper catalysts for degradation of p-chlorophenol. Catal Today 124:224–231. https://doi.org/10.1016/j.cattod.2007.03.040
Lyngsie G, Krumina L, Tunlid A, Persson P (2018) Generation of hydroxyl radicals from reactions between a dimethoxyhydroquinone and iron oxide nanoparticles. Sci Rep 8:10834
Mahamuni NN, Adewuyi YG (2010) Advanced oxidation processes (AOPs) involving ultrasound for waste water treatment: a review with emphasis on cost estimation. Ultrason Sonochem 17:990–1003
Nidheesh PV, Ganiyu SO, Martínez-Huitle CA, Mousset E, Olvera-Vargas H, Trellu C, Zhou M, Oturan MA (2023) Recent advances in electro-Fenton process and its emerging applications. Crit Rev Environ Sci Technol 53:887–913. https://doi.org/10.1080/10643389.2022.2093074
Ragonese R, Macka M, Hughes J, Petocz P (2002) The use of the Box-Behnken experimental design in the optimisation and robustness testing of a capillary electrophoresis method for the analysis of ethambutol hydrochloride in a pharmaceutical formulation. J Pharm Biomed Anal 27:995–1007. https://doi.org/10.1016/s0731-7085(01)00659-8
Sastry SV, Khan MA (1998) Aqueous based polymeric dispersion: Plackett–Burman design for screening of formulation variables of atenolol gastrointestinal therapeutic system. Pharm Acta Helv 73:105–112. https://doi.org/10.1016/s0031-6865(97)00052-6
Sounni F, Aissam H, Ghomari O, Merzouki M, Benlemlih M (2018) Electrocoagulation of olive mill wastewaters to enhance biogas production. Biotechnol Lett 40:297–301. https://doi.org/10.1007/s10529-017-2464-5
Zarei M, Khataee AR, Ordikhani-Seyedlar R, Fathinia M (2010) Photoelectro-Fenton combined with photocatalytic process for degradation of an azo dye using supported TiO2 nanoparticles and carbon nanotube cathode: neural network. Electrochim Acta 55:7259–7265. https://doi.org/10.1016/j.electacta.2010.07.050
Zhong X, Royer S, Zhang H (2011) Mesoporous silica iron-doped as stable and efficient heterogeneous catalyst for the degradation of C.I. Acid Orange 7 using sono-photo-Fenton process. Sep Purif Technol 80:163–171. https://doi.org/10.1016/j.seppur.2011
Acknowledgements
I would like to thank Prof. Dr. Nuri Azbar, a faculty member of Ege University Bioengineering Department, for his support.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Contributions
EC contributed to the design of the experiments, the collection of wastewater, and the evaluation of experimental results and writing of the article. SE contributed to the evaluation and writing phase of the experimental results. ET contributed to the experiments.
Corresponding author
Ethics declarations
The authors declared that they have no conflict of interest.
Additional information
Editorial responsibility: S.Mirkia.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Çokay, E., Eker, S. & Taşkın, E. Application of the sono-Fenton/UV process on the treatment of table olive processing wastewater. Int. J. Environ. Sci. Technol. (2024). https://doi.org/10.1007/s13762-024-05926-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13762-024-05926-9