Abstract
The sorption of zinc chloride ions from hydrochloric acid-based solutions using anionic resins was investigated. A two-stage experiment was planned. In the first stage, parameter optimization was performed using the Taguchi experimental optimization approach for type of anionic resins, initial Zn (II) ion concentration, resin dose, agitation rate, and temperature and time of the process as process parameters. According to the signal—to—noise ratio values calculated with the larger better-quality feature; the optimum parameter levels were determined as chloride form resin, 10 g/L, 1200 mg/L, 200 rpm and 35 °C and 90 min, respectively. Under optimum operating conditions, the sorption capacity of the resin for zinc chloride complex ions was 46.52 mg/g. The energy dispersive X-Ray analyses confirmed that zinc chloride complexes bind to the resin’s surfaces. In the second step, equilibrium and kinetic tests were performed under the optimum parameters. The tests results were compared with six equilibrium isotherm models with 2 or more parameters and four kinetic models. The non-linear solution approach was applied for all models. Langmuir isotherm and the general order models were the best fit. The values of \({K}_{L}\) and \({q}_{m,L}\) for the Langmuir model were 2.15*10–3 L/g and 66.08 mg/g, respectively. The kinetic model can be given by an equation of order 3.26. Accordingly, \({k}_{n}\) and \({q}_{e,n}\) were 2.18*10–5 min/(g mg)2.26 and 60.03 mg/g, respectively. The process mechanism was a typical chemical sorption. According to the results of desorption tests conducted using 1 M HCl, the desorption efficiency was 65%.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
The hot-dip galvanizing is the process of coating the steel surface with zinc. One of the most important steps of the process, also known as pickling, is the cleaning of iron oxide on the steel surface with strong hydrochloric acid solutions (Hu et al. 2022). The waste liquor released in large quantities during the pickling process is known as spent pickling liquor. The spent pickling liquor contains traces of metals such as lead, nickel, copper and manganese, as well as high concentrations of zinc and iron. Therefore, its disposal is very important from an environmental and economic perspective (Sinha et al. 2014).
Many physicochemical approaches such as adsorption, biosorption, ion exchange, coagulation-flocculation, precipitation, membrane separation, flotation and solvent extraction can be used for zinc removal or recovery from wastewater (Abidli et al. 2022). However, when zinc is present in acid-based environments such as spent pickling solution, only ion exchange, electrodialysis, solvent extraction and chemical precipitation processes can be applied from these approaches (Pietrelli et al. 2018). The ion exchange has been used effectively and successfully for the removal and recovery of heavy metals from industrial wastewater.
In studies investigating the removal or recovery of zinc using ion exchange resins, cation exchange or chelate resins have been tested more than anion exchangers, probably due to the ionic type of zinc in solution (Kononova et al. 2011). However, the performance of strong base (SBA) or weak base (WBA) anion exchange resins in removing zinc (II) complexes from chlorinated solutions has been tested by some researchers.
Miesiac (2005) obtained a zinc chloride removal capacity between 0.62–0.76 mol/L in his study using Lewatit’s strong and/or weak basic VPOC1071, MonoPlus M500 and MonoPlus MP64 resins in an ion exchange column. In a study using Purolite A500, Purolite S985, AV-17–8 and intermediate base (IBA) AM-2B as anion exchange resin, the recovery of zinc ions from chloride and chloride-sulphate solutions was tested (Kononov 2011). For an initial Zn (II) concentration of 1 mmol/L, a removal efficiency of 70—78% was obtained. In another study, the removal of zinc chloride complex ions in extremely environments using SBA Amberlite IRA410, Purolite NRW700, Purolite A103S and Purolite A400MBOH resins was investigated (Gîlcă et al. 2014). The highest experimental ion exchange capacity was obtained for Amberlite IRA410 as 8.34 mg/L. Wołowicz (2019) investigated the removal of Zn (II) complex ions from both model chloride and chloride-nitrate(V) solutions. Experiments were carried out for zinc concentrations of 100 mg Zn (II)/L at different HCl concentrations using SBA Dowex PSR2, Dowex PSR3, Purolite A400TL and Lewatit MonoPlus SR7, chelating type Lewatit MonoPlus TP220 and Purolite S984, and WBA Purolite A830 anionic resins. The highest sorption capacity was obtained for Lewatit MonoPlus TP220, Purolite A400TL and Lewatit MonoPlus SR7 resins as 9.5 mg/g in 3 M HCl solutions.
There are no examples in the literature regarding the application of Purolite PFA400MB for the removal and sorption of zinc chloride complexes. In this study, the performance of both Purolite PFA400MB and strong base anion exchanger Purolite A400MBOH resin has been tested for this purpose. These SBA resins, in type 1 and gel form, have a styrene–divinylbenzene copolymer matrix functionalized with quaternary ammonium. Although the physical and chemical properties of the resins produced for demineralization applications are similar, the ionic form of Purolite PFA400MB is in the Cl− form and the other is in the OH− form.
As in many sorption studies for the removal/recovery of heavy metals, in the current study, Taguchi's experimental design and parameter optimization tool was used as a systematic approach that can effectively reveal the effects of multiple factors in terms of time and cost management (Zolfaghari et al. 2013; Ali et al. 2021; Dakhem et al. 2022; Inès et al. 2022). Taguchi process optimization is an approach that uses orthogonal arrays to estimate the effects of each parameter and its levels on the variation of the response (Davis & John 2018). “Orthogonal Arrays” (OA) allow any effects to be investigated independently from others and can also enable data analysis and prediction of best results as measurable functions for optimization (Karoke & Jadhao 2021).
This study aimed to test the ion adsorption performances of zinc chloride complexes from strongly acidic environments under equilibrium ion exchange conditions using Purolite PFA400MB and A400MBOH SBA resins. In the study, first of all, the optimization of process parameters such as process temperature and time, initial ion concentration, resin type and dose, and agitation rate were carried out using Taguchi's experimental design. Equilibrium ion adsorption and kinetic tests were then conducted at optimum parameter levels. Experimental data were analysed using a nonlinear approach for equilibrium ion adsorption isotherm and kinetic models. Additionally, regeneration performance tests were conducted for the optimum resin type.
Materials and methods
Resins and reagents
A400MBOH and PFA400MB strong base anion resins were supplied by Purolite. Both resins are copolymers of cross-linked polystyrene with divinylbenzene and functionalized by quaternary ammonium. The resins A400MBOH (Purolite 2023a, b) and PFA400MB (Purolite 2023a, b) have OH and Cl, respectively, as the counter ion. Their appearances are light yellow spherical beads. The other physical and chemical properties are reported on the supplier's website.
To prepare 2000 mg/L zinc stock solution, the required amount of Merck grade zinc (II) chloride (ZnCl2) salt was dissolved in 3 M HCl. Then, the stock solution was diluted with 3 M HCl in appropriate proportions and used in the experiments. The 3 M HCl solutions were also prepared with deionized water using Merck grade hydrochloric acid.
Apparatus
The samples were analysed using a PerkinElmer AAnalyst 400 AAS flame and graphite-furnace atomic absorption spectrometery (AAS). The detection limit of AAS for Zn (II) ion is 0.0015 mg/L. The amount of metal remaining in the solution phase was analysed. The results of measurements repeated three times were averaged. The maximum standard deviation among replicate results was 1.0%.
Scanning electron microscopy (Quanta FEG 250) operating at 15 kV has been used to study the micro surfaces of the resins. Their composition and element contents before and after sorption were analysed by using energy-dispersive X-ray spectroscopy (SEM/EDAX).
Experimental procedure
The experiment studies consisted of two parts: In the first part, were performed batch sorption tests for Taguchi experimental optimization. In the second section, batch sorption tests were carried out to observe the ion exchange mechanism by using isotherm and kinetic models under the optimum parameter levels determined from the experimental optimization studies.
The tests were carried out in a thermostatic orbital shaker. 50 ml of zinc chloride solution and a given amount of the resin were added to 250 ml stoppered glass Erlenmeyer flasks. All process parameters were adjusted at the given level. At end of a given processing time, the supernatants were filtered through filter papers (0.45 μm Whatman) and analysed the Zn (II) using by AAS.
The mass (mg) of zinc chloride complex ions sorbed by 1 g of the resin mass at equilibrium is calculated as follows:
Experimental design and optimization
In order to determine the optimum conditions of the sorption process with a minimum number of experiments, L18 (2135) was applied. The theoretical background regarding the Taguchi experimental design method is well known in the literature and is also given in the Supplementary file of this study.
For this study, the resin type, initial Zn (II) concentration, process temperature, resin dose, agitation rate and process time have been considered as controllable factors. These are indicated by A, B, C, D, E and F respectively. The L18 (2135) design matrix and parameter levels followed in the experiments are given in Table 1. As shown in Table 2, the resin type is arranged in two levels (L1 and L2) and the other factors in three levels (L1, L2 and L3). The parameter levels were determined based on previously conducted preliminary tests. Each test was repeated twice under the same conditions, taking into account the effects of uncontrollable factors. The mean of the sorption capacities calculated with Eq. 1 was recorded as the "response" in the orthogonal matrix.
One of the most important advantages of the orthogonal arrays is the reduction in the number of experiments. Considering the parameters and their levels presented in Table 2, a five-factor full factorial experimental design with three levels each for the two resin types would include a total of 2*35 (486) experiments. The orthogonal array reduced the number of experiments from 486 to 32 (2*16 in two repetitions), thus significantly reducing time and cost.
In Taguchi's orthogonal arrays method, the evaluation of test results is based on the signal-to-noise ratio (S/N). Since the conditions in which Zn (II) removal from acidic solution is maximum were determined in this study, the "bigger is better" option as a quality characteristic was considered. Additionally, ANOVA (analysis of variance) was performed to determine the effect of each parameter on the overall efficiency of the process. The orthogonal array design matrix was created using Minitab® 20.3 software. The results of the tests were then entered into the software and the evaluation was conducted.
Equilibrium and kinetic tests
While performing the equilibrium and the kinetic tests, the optimum parameter levels obtained from the Taguchi experimental design were considered in the first section. Under these optimum levels (T = 35 °C, m = 10 g/L, agitation rate = 200 rpm and using PFA400MB), the equilibrium tests were performed at C0 values of 300 – 1400 mg/L and the sorption time of 90 min. The kinetic tests were conducted at a C0 value of 1200 mg/L at the sorption times ranging from 1 to 90 min.
Data management
In many sorption processes, a thermodynamic equilibrium relationship is assumed between the amount of sorbate adsorbed per unit mass of sorbent at a constant temperature and the amount of sorbate remaining in the liquid phase, and isotherm models are developed based on these assumptions (Saadi et al. 2015). Therefore, the relationship between the amount of zinc chloride sorbed on the resin and the concentration of zinc chloride dissolved in the solution at equilibrium can be given by adsorption isotherms. This relationship can also give an idea about the sorption mechanism. On the other hand, sorption kinetic profiles are also important for sorption processes as they can provide useful information about sorption rate, sorbent performance and diffusion or reaction base mechanisms. (Wang & Guo 2020).
In this paper, six conventional equilibrium isotherm models with two or more parameters, which are widely used in the literature, are applied to experimental equilibrium data: two-parameter Langmuir and Freundlich, three-parameter Redlich-Peterson and Sips models, four-parameter Baudu model and five parameter Fritz-Schlunder model. Also, experimental kinetic data are compared with Pseudo—1° order (PFO), Pseudo—2° order (PSO), General order and Elovich models. The equations of the applied isotherm and kinetic models are given in the Supplementary file Table 1. Details on the development and implementation of these models can be found in the literature (Saadi et al. 2015; Wang & Guo 2020).
The non-linearized equations of the equilibrium and kinetic models were fitted using the Microsoft Solver add-in in Excel and the correlation coefficients (R2, Radj2) and sum of squares errors function (SSE). Mathematical expressions of the functions are presented in Supplementary file Table 2.
Regeneration tests
The regeneration of the sorbent material is an important parameter regarding the application potential of sorption processes.
The sorption process was carried out between the Purolite PFA400MB resin and the solution having an initial Zn (II) concentration of 1200 mg/L. The exhausted resin was filtered and Zn (II) in solution was analysed. To remove excess zinc chloride between the resin pores, it was first washed with deionized water and then treated with 1 M HCl solution to desorb the zinc chloride complex ions and reuse the resin. At the end of the regeneration process, the resin was filtered again, washed with deionized water and analysed for Zn (II) in solution. For both sorption and regeneration processes, the contact time and the agitation rate were set to 90 min and 200, respectively. The sequential sorption-regeneration processes were repeated five times. The removal efficiency, was also calculated from the batch experiments and defined as follows:
Results and discussion
Parameter optimization
The effects of the factors consisting of anionic resin type, initial Zn (II) concentration, process temperature, resin dose, agitation rate and process time and their selected levels on the removal capacity of zinc chloride ions from acidic solutions were studied. The tests were performed in duplicate in accordance with the L18 (2135) orthogonal array. The mean adsorption capacity calculated from the tests was entered into the orthogonal matrix as the response value. The S/N ratios were calculated under "bigger is better" conditions as the quality characteristic in evaluating the results of the experiments. The mean response values (qe) determined from the experiments performed appropriately the experimental design matrix (Table 1) and the calculated S/N ratios are given in the Supplementary File Table 3. The Run 9 was found to have the highest S/N ratio and average sorption capacity with values of 33.21 and 45.75 mg/g, respectively, but for the Run 10, these variables showed their lowest values as 25.15 and 18.10 mg/g, respectively.
The optimum level for each process parameter is the level corresponding to the biggest S/N ratio for "bigger is better" characteristic. The response and S/N ratios curves for the individual effects of zinc chloride sorption process parameters are shown in Fig. 1. These curves can show changes in values of the S/N ratios and the mean response versus the parameter levels. The S/N ratios and rank values, which enable the parameters and their levels to be arranged according to their effect sizes, are given in the Supplementary File Table 4. Delta is a value that measures the magnitude of the effect of a factor and also reveals the relative impact of the effect. It bases this on the difference between the highest and lowest characteristics of the factor's mean. The intensity of the effect is parallel to the height of the difference. By the rank value, the factors with the relative largest impact can be determined (Fernández-López et al. 2019).
The levels of parameter strongly affected the sorption capacity. Additionally, the largest and smallest factors affecting the S/N ratio were D (the resin dosage) and C (the process temperature), respectively. The effects of the parameters according to the rank values were: D (the resin dose) > A (the resin type) > B (the initial Zn (II) concentration) > E (the agitation rate) > F (the proses time) > C (the process temperature).
The process parameters that enable the resin to reach its maximum adsorption capacity were determined as follows: at the D1 (10 g/L) for the resin dose, at the A1 (PFA400MB) for the resin type, at the B3 (1200 mg/L) for the initial Zn (II) concentration, at the E1 (200 rpm) for the agitation rate, at the F2 (90 min) for the proses time, and at the C3 (35 °C) for the process temperature. Thus, the optimum combination of the process parameters was 10 g/L of the anionic resin PFA400MB, the temperature of 35 °C, the agitation rate of 200 rpm, the initial Zn (II) concentration of 1200 mg/L and 90 min of time. A batch process run under these optimum process parameters can be expected to show the highest sorption capacity for zinc complex chloride ions removal.
Analysis of variance for the parameter’s optimization
ANOVA was performed to determine the effect of each process parameter on the sorption capacity. This analysis revealed the percentage contribution of each parameter at a 95% confidence interval, which also ensures the reliability of process optimization (Taguchi 1986). The results of the ANOVA analysis are reported in the Supplementary File Table 5.
The F-ratio and p-value in the table of ANOVA have been calculated to examine the statistical significance between the parameters. The F-ratios obtained are used for determining the statistical significance of the process parameter. A larger F-ratio indicates that change of a parameter causes a large change in the performance. The contribution of each parameter to qe is given in Fig. 2. It can be concluded that resin dose and type are the most effective factors on the response. Their contributions percentage have been calculated to be about 48.26 and 30.26%, respectively. The parameter with the third significant effect on the sorption efficiency is the initial Zn (II) ion concentration with 13.63%. When compared with the Fischer table F-ratios, the calculated F-ratios for resin type and dose are greater than the Fischer table's F-ratios of F0.05,1,6 = 5.9874 and F0.05,2,6 = 5.1433, respectively. As with that of the initially Zn (II) ion concentration. In addition, p values for resin type and dose have been found to be less than 0.005 and for initial Zn (II) ion concentration less than 0.05. According to these results, the relationship between the sorption capacity and these parameters is also significant in terms of statistical significance.
A percentage error contribution of less than 50% is considered to mean that the experiments were performed under controlled conditions (Googerdchian et al. 2018), which in this study was determined to be only 4.97%. It was observed that the effects of process temperature and the agitation rate on the sorption capacity were quite low.
According to "bigger is better” quality characteristic analysis (the Supplementary File Table 3) and Fig. 2, the most important factor contributing to the sorption performance of zinc chloride complexes was the resin dose. According to Fig. 1, the mean sorption capacity and the S/N ratio decreased as the resin dose increased from 10 to 30 g/L.
Increasing the number of active sites as the resin dosage increases causes the concentration of zinc chloride complex ions on the resin surface to increase. Thus, a decrease in the concentration gradient between the concentrations of zinc chloride complex ions in the bulk solution and on the resin surface, and hence the equilibrium sorption capacity (mg/g), is observed. However, despite the decrease in sorption capacity (mg/g), an increase in removal efficiency (%) may occur. In order to control this, the main effects of the factors have been re-plotted depending on the removal efficiency (the Supplementary file Fig. 1). In this way, it has been clearly seen from the replotting that the removal efficiency increased with the increase of resin dosage. The results of the effects of resin dosage on the process agree with those found for ZnCl4−2 sorption on Amberlite IRA410 resin by Gîlcă et al. (2017). According to some other researchers reporting the same trend; increasing the amount of resin in a fixed volume may: (1) create electrostatic interferences, reducing the force of attraction between the electrical surface charges on closely spaced resin particles and the adsorbed solute. (2) the particles may create a physical blockage for the adsorbed solute, thus causing a decrease in adsorption (Arias & Sen 2009; Wang et al. 2013).
According to the results in Fig. 2, the second most important factor affecting the sorption of zinc chloride complex ions from the HCl-based solution was the type of anionic resin with a contribution of 30.26%. Taguchi's optimization tool based on larger better-quality characteristic produced a larger S/N ratio for SBA resin PFA400MB in the form of Cl than for A400MBOH resin. Therefore, it can be considered that the PFA400MB anionic resin has a higher sorption capacity, which agrees with similar studies (Gîlcă et al. 2014; Wołowicz 2019) on the removal of zinc chloride complex ions using SBA and WBA anion exchange resins with different ionic forms under strongly acidic conditions.
According to the results given in Fig. 2, the initial Zn (II) concentration significantly affected the zinc chloride the sorption with a contribution of 13.63%. As the initial Zn(II) concentration increases from 800 to 1200 mg/L, the S/N ratio tends to increase, which is a result consistent with similar studies in the literature (Zhou et al. 2019). Essentially, increasing the concentration of the adsorbed ion can cause an increase in the adsorption capacity to some extent (Mohan et al. 2017). Because the increase in the initial ion concentration causes an increase in the mass transfer driving force, and therefore the interaction of the adsorbed ions with the resin surface and the ion adsorption capacity may also increase (Xinyu et al. 2022). This is the reason for the mismatch between the sorption capacity and the removal efficiency in the initial ion concentration, similar to that seen in the resin dose.
The agitation rate and the process temperature seem to be factors that affect the sorption capacity at relatively lower with the contributions of 1.7 and 0.85%, respectively.
The proses time was parameter the lowest effect the sorption of zinc chloride complex ions on the resin. But it was also found that the S/N ratio was slightly larger in the 90-min the level of time. In the preliminary experiments carried out on the sorption of zinc chloride complex ions from HCl-based solutions by the resins, it was observed that the sorption capacities reached approximately 91–95% of the equilibrium sorption capacity in a process time of 60 min.
The agitation rate and the process temperature seem to be factors that affect the sorption capacity at relatively lower with the contributions of 1.7 and 0.85%, respectively. The S/N ratios were determined to be some larger for the agitation level of 200 rpm and the temperature level of 35 °C. According to these levels of parameters the sorption capacity increased as the agitation rate decreased and the temperature increased. The increase in sorption capacity versus low agitation rate is due to proper resin dispersion throughout the solution. However, the high agitation rate may cause the suspension not to remain homogeneous due to the eddy phenomenon (Tang et al. 2017; Bilal et al. 2021). This may also result in the zinc chloride complex ions not sorbed to the resin surface and higher sorption capacities are not observed.
Temperature is parameter that can affect its sorption by affecting its solubility and interactions of resin and zinc chloride complex ions (Peng et al. 2017). The increase in the sorption capacity relatively with increasing temperature may be due to increased diffusion of the ions at high temperatures, and may also indicate that the sorption reaction occurs spontaneously and is endothermic in nature (Duan et al. 2020).
The Taguchi orthogonal array approach requires confirmation experiments for optimum quality characteristics. For this, the confirmation experiments were carried out under the optimum parameter levels A1, B3, C3, D1, E1 and F2. The S/N value corresponding to the mean sorption capacity was calculated based on the "bigger is better" quality characteristic and compared with the predicted values. The mean sorption capacity and S/N ratio were estimated by the software to be 43.32 and 33.09, respectively, within the 95% confirmation interval (38.28 < mean < 48.37 and 32.06 < S/N < 34.13). In the confirmation tests, these values were observed as 46.52 and 33.35, respectively. Therefore, it can be also considered that the results of experimental are reproducible.
The observed results are compatible with the predicted results of the linear regression modelin the L18 (2135) design matrix based on all tests. The predicted and the observed results can be described by a line with an intercept of 0.000 and a slope of 1.000 (R2 = 0.95), within the 95% confidence interval. The model graph is given as Fig. 2 in the Supplementary File.
Equilibrium isotherm models and profiles
McMahon et al. (2019) investigated Zn (II) complex species at 10–3 and 10–6 mol/L concentrations in chlorinated solutions and reported their fractions depending on the Cl− concentration and pH in the solution (McMahon et al. 2019). Accordingly, in solutions with high chloride content (5.45 mol Cl−/L), ZnCl4−2 was determined as the dominant species in the pH -1 to 9.8 range. This was followed by ZnCl3− and relatively less ZnCl20 (an ion fraction of about 0.06) was present.
PFA400MB has a styrene—divinylbenzene copolymer matrix, functionalized with quaternary ammonium, and also Cl ionic form. During processing, the reactions between the PFA400MB resin and the ionic species ZnCl4−2 and ZnCl3− probably involve the two mechanisms (orange circles to indicate the styrene—divinylbenzene copolymer matrix) in Eq. (3) and (4), respectively.


Although the ionic fraction of ZnCl20 is quite low under strongly acidic conditions, its sorption on the PFA400MB resin may also be possible. Wołowicz & Hubicki (2016) propose the following two-step neutral species mechanism (Eq. 5 and 6) for the sorption of ZnCl20 by an anionic resin, which they attribute to Horne et al. (1957).


Equilibrium sorption tests were performed under the following optimum parameter levels provided by the Taguchi experimental design tool: T = 35 °C, m = 10 g/L, agitation rate = 200 rpm and resin type = PFA400MB.
Isotherm models are necessary both to reliably estimate sorption parameters and to determine quantitatively the optimal sorption equilibrium behaviour of the resin for different experimental conditions. This modelling is important to define not only the sorption mechanism but also the sorbent surface properties (Wasewar et al. 2008). In this study, sorption isotherms were used to determine the equilibrium distribution of zinc chloride ions between the resin and bulk phases. The data from batch tests were compared with two, three, four and five parameter equilibrium adsorption isotherm models to determine the best-fitting model. The model parameters were determined from nonlinear solutions of Langmuir, Freundlich, Redlich-Peterson, Sips, Baudu and Fritz-Schlunder adsorption isotherm models.
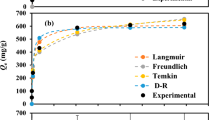
The model parameters produced from the nonlinear solutions of the model equations for the largest values of R2, and Radj2 and the smallest values of SSE are given in Table 3, and the isotherm and experimental profiles are given in Fig. 3. The Fig. 3 presents the observed and expected profiles of the adsorption capacity of the resin versus the concentration of zinc chloride ions remaining in the equilibrium solution. In the comparison of experimental data with equilibrium isotherm models, R2, Radj2 and SSE were taken as evaluation criteria. The nonlinear solution produced values of R2 greater than 0.98 for all models. For Radj2 and SSE, the ranking was as follows:
For Radj2; Langmuir > Sips > Redlich-Peterson > Baudu > Freundlich > Fritz-Schlunder.
For SSE; Langmuir < Baudu < Sips < Redlich-Peterson < Fritz-Schlunder < Freundlich.
Accordingly, the Langmuir model was the model that best fitted the experimental data. The agreement between the observed data and the Langmuir Fit curve is also clearly seen in Fig. 3. For the sorption of zinc chloride complex ions from the acidic solutions by the PFA400MB resin, the model estimated the maximum sorption capacity and the Langmuir equilibrium constant KL as 66.08 mg/g and 2.15*10–3 L/g, respectively.
The Langmuir isotherm model assumes that adsorption occurs in a one-molecule-thick monolayer on a homogeneous surface. According to this empirical model, sorption can occur in the same, equivalent, and finite number of localized regions. All active sites on the homogeneous surface have equal affinity for the adsorbate (Foo and Hameed 2010). In fact, most of the studies reported in the literature for the sorption of heavy metals from aqueous solutions are monolayer chemisorption processes (Xinyu et al. 2022). Similar to this study Zhou et al. (2019) also reported that the Langmuir model is the model that best fits the experimental data in their study for the removal of Zn (II) from manganese and zinc chloride wastewater by ion exchange.
According to the results presented in Table 3, the experimental results provided the best fit to the Sips and the Redlich-Peterson models in terms of the Radj2 values and the Baudu model in terms of the SSE value, after the Langmuir model. The Baudu isotherm model assumes that the Langmuir coefficients (\({q}_{m,L}\) and KL) are not constant over a wide range of concentrations, and the equation can be applied in the range (1 + x + y) < 1 and (1 + x) < 1 (Saadi et al. 2015; Xinyu et al. 2022). The closer the x and y values, known as Baudu isotherm exponents, to zero, the closer the model to the Langmuir model. The non-linear solution of Baudu's equation calculated the values of the x and y exponents as 1.533*10–4 (quite close to zero) and 0.000, respectively. Therefore, the model parameters (q and K), R2 and SSE values are very close to the values produced for the Langmuir isotherm model. The slight difference between the Radj2 values (0.985 and 0.990) of both models is due to the fact that Baudu model has four parameters and Langmuir model has two parameters.
According to the results given in Table 3, the non-linear solution of the Redlich-Peterson equation produced the 0.988 value of Radj2 and the 5.560 value SSE. These statistics predicted the value of the \({\beta }_{RP}\) exponent as 0.9008, close to 1. Other model parameters \({K}_{RP}\) and \({\alpha }_{RP}\) were estimated as 0.1594 L/g and 0.0050 (1/mg) 0.9008, respectively. For the Sips equation was produced the value of Radj2 of 0.989 and the SSE of 5.127. The model estimated the Sips maximum sorption capacity \({q}_{m,S}\) and the equilibrium constant \({K}_{S}\) to be 76.33 mg/g and 0.0033 (L/g)0.882, respectively. The heterogeneity factor \({n}_{S}\) was again close to 1 as 0.882.
Both of the Redlich-Peterson and Sips model incorporate the Langmuir and Freundlich isotherm models. The Redlich-Peterson model can be applied to homogeneous and heterogeneous systems. In this model, the exponent, \({\beta }_{RP}\), can take values between 0 and 1. For \({\beta }_{RP}\) =1, it is assumed that the model is reduced to the Langmuir adsorption isotherm model, which indicates monolayer adsorption (Foo and Hameed 2010). The Sips adsorption model is reduced to the Freundlich isotherm model at low adsorbate concentrations, but it can predict the monolayer adsorption parameters of the Langmuir isotherm at high adsorbate concentrations. In this isotherm, the greater the value of the \({n}_{S}\) exponent, called the heterogeneity factor, is considered to be the greater the heterogeneity of the system. At \({n}_{S}\)=1, the sorbent in the process is considered to have an ideal surface and the conditions of the monolayer Langmuir adsorption mechanism are valid. As a general rule, the model parameters are affected by the variation of process parameters such as pH, temperature, and concentration (Foo and Hameed 2010; Saadi et al. 2015).
The Freundlich and the Fritz-Schlunder isotherm models were the models that showed the least agreement with the results of the equilibrium tests (Table 3 and Fig. 3). According to their non-linear solutions, the SSE errors (12.155 and 12.154) were close to each other and larger than the other models, but the Radj2 data of 0.979 of the Freundlich isotherm model and 0.957 of the Fritz-Schlunder isotherm model were significant.
The Fritz-Schlunder isotherm model, a five-parameter empirical model, is derived from incorporate the Langmuir and Freundlich isotherm models. This model approaches the Langmuir isotherm model when \({m}_{1,FS}\) and \({m}_{2,FS}\)=1, and the Freundlich isotherm model at high concentrations of sorbate (Saadi et al. 2015). The maximum equilibrium capacity was estimated as 93.61 mg/g from the Fritz-Schlunder equation. In addition, the equilibrium constants \({K}_{1,FS}\) and \({K}_{2,FS}\) and the exponents \({m}_{1,FS}\) and \({m}_{2,FS}\) of the model were predicted as 0.0153, 0.0498, 0.5147 and 0.0196, respectively. The Freundlich heterogeneity coefficient, \({n}_{F}\) was estimated as 1.947 for the sorption of zinc chloride complexes from strongly acidic solutions at equilibrium conditions using the PFA400MB resin. The Freundlich isotherm model describes monolayer and multilayer adsorption, respectively, depending on whether the primary adsorption mechanism in an adsorption process is chemisorption or physisorption. For the heterogeneity factor \({n}_{F}\), the process is considered favourable if 1/\({n}_{F}\) is 0 < 1 /\({n}_{F}\) < 1, if it is greater than zero, the process is unfavourable, and if equal to one, it is considered irreversible (Xinyu et al. 2022). Gîlcă et al. (2014) in their study investigating the sorption of zinc on Amberlite IRA410 at equilibrium conditions, reported that the sorption data fit well with the Freundlich model and they found the \({n}_{F}\) value as 1.29.
Sorption kinetic models and profiles
The PFO, the PSO, the General order and the Elovich kinetic models (the Supplementary file Table 1) were used to define the mechanism of zinc chloride complexes sorption by the PFA400MB resin. The possible effects of temperature on sorption kinetics were investigated separately.
The pseudo-first order equation, also known as the Lagergren equation, has generally been the widely used model for modelling liquid sorption. This model is used when the number of occupied bond sites on the resin is proportional to the number of unoccupied sites (Vergili et al. 2013). If the PFO kinetics does not calculate process kinetics correctly then the PSO kinetics can be applied. The PSO model assumes that the number of occupied binding sites is proportional to the square of the number of unoccupied sites. The PSO kinetics agrees with chemisorption as the rate control step (Ho and McKay 1999). The general order model, on the other hand, proposes the application of the universal rate law to a chemisorption process without any further assumptions. According to this kinetic approach, regardless of the coefficients of the chemical reaction equation in a process, the number of occupied binding sites can be proportional to the number of unoccupied sites, as an integer or non-integer rational degree (Wagner et al. 2012). In addition, the Elovich model has been also used because it describes chemisorption well (Wang and Guo 2020).
The parameters calculated from the kinetic models are presented in Table 4 and also Fig. 4 illustrate graphs that take temperature into account. It is clearly seen from the profiles in Fig. 4 that the sorption increases as the temperature increases. As shown in Fig. 4, the sorption edge formed rapidly in about 20 min, but the sorption proceeded more slowly until equilibrium was reached in 90 min. Surface sorption, which occurs easily in the active areas on the resin surface, can enable the process to progress rapidly in approximately 20 min. As zinc chloride ions bound to the active sites, the sorption process slowed down and sorption rates decreased due to the decrease in the diffusion rate. The results show that regardless of the temperatures tested, the sorption process reached equilibrium in approximately 90 min.
The R2, Radj2 and SSE are used to quantitatively compare the applicability of different kinetic models. A higher R2 (0.932–0.996) and Radj2 (0.913–0.994) and a lower SSE (5.32 – 106.63) represent better fit. It can be said that the PFO and Elovich models are the models that show the lowest agreement with the experimental results.
The general order model is best fitted to explain the sorption kinetics of zinc chloride complex ions at all temperatures. Depending on temperature increases of 298, 303 and 308 K, it can be said that the number of occupied binding sites on the resin surface is proportional to 1.99, 2.65 and 3.26 degrees (n) of the number of unoccupied sites, respectively. For n = 2, the general order model is reduced to the PSO model. In this study, the predicted \({k}_{n}\) (2.47*10–3 min/(g·mg) 0.99) and \({q}_{e,n}\) (50.10 mg/g) values of the general order model for 1.99 degrees are quite close to the \({k}_{2}\) (0.0024 g/mg·min) and \({q}_{e,PSO}\) (50.17 mg/g) values predicted by the PSO model at 298 K. With increasing temperatures, the rate constant (\({k}_{n}\)) of the general order model decreased from 2.47*10–3 min/(g·mg)0.99 to 2.18*10–5 min/(g·mg)2.26 depending on the increase in n. However, it was observed that the initial sorption rate (h) and the sorption capacities (\({q}_{e,n}\)) increased with increasing process temperature. This trend indicates that the sorption of zinc chloride complex ions onto the PFA400MB resin is a chemisorption, the chemical reaction is dominant in the rate controllling step of the process (Ho & McKay et al. 1998) and the process is endothermic nature (Vergili et al. 2013).
Surface morphology and EDAX Analysis
SEM/EDAX analyses were conducted for Purolite PFA400MB and A400MBOH anionic resins to determine their morphology and elemental mass ratios before and after the sorption. In Figs. 5 and 6, the morphological differences on the surface of the resins before and after zinc chloride complexes loading can be seen with the SEM micrographs presented at 250 × and 16 kx magnification, and the changes in the compositions and elemental contents of the resins can be seen with the EDAX spectra.
Homogeneous surface morphologies of the PFA400MB and the A400MBOH resins before zinc chloride complexes loading are seen at 250 × and 16 kx magnifications in Figs. 5a, b, and 6a, b, respectively. On the other hand, it can be clearly seen that the resin surfaces have a saturated and rougher morphology, especially from the 16 kx magnification images (Figs. 5e and 6e) after zinc chloride complexes loading.
While the presence of C, N and O is observed on the pure surface of the A400MBOH anion exchange resin (Fig. 6c), the presence of Cl with these ions is also observed on the pure surface of the PFA400MB anion exchange resin in Cl form (Fig. 5c). EDAX spectra after zinc chloride complexes loading (Figs. 6f and 7f) show characteristic peaks for Zn at 1.012 keV and 8.634 keV. The characteristic Cl peak at 2.622 keV is sharper in the PFA400MB resin loaded in Fig. 5f than in its pure state in Fig. 5c and is also present in the spectra of the loaded A400MBOH resin in Fig. 6f. This confirms that zinc chloride complex ions bind to Purolite PFA400MB and A400MBOH anionic resins.
Regeneration
The use of ion exchange resins in wastewater treatment depends not only on the sorption capacity but also on resin regeneration and recycling. Because in addition to keeping the total cost of the treatment process to a minimum, it may also be important to recover the sorbed ions from the resin surface. Although there is a linear decrease in the removal efficiency of the resin after repeated sorption–desorption cycles, the regeneration process can still provide high sorption capacity and reuse values (Bilal et al. 2021).
After sorption of zinc chloride complex ions on the PFA400MB resin, the regeneration performance of the resin was tested. For this, after equilibrium sorption process with 1200 mg/L initial Zn (II) solution, equilibrium desorption process was carried out using 1 M HCl as eluting agent. In the equilibrium desorption process, the sorption mechanism given by Eqs. 3 and 4 can be expected to be reversed by Eqs. 5 and 6 as follows:
The results of the sorption—desorption tests repeated as five cycles are compared in Fig. 7. The efficiency of sorption of zinc chloride complex ions of the PFA400MB resin ranged from about 34 to 29%, while the efficiency of desorption with 1 M HCl decreased from 65 to 33%, about half.
In a similar study, after zinc chloride complexes sorption on SBA Purolite A400TL and Lewatit MonoPlus SR7 resins in Cl form, desorption process was carried out using HNO3, HCl, NH3H2O, NaOH, H2SO4 and NaCl as eluting agents. In sorption–desorption studies carried out as three cycles, sorption tests were performed for an initial concentration of 100 mg/L Zn (II) (in 0.1 M HCl). Sorption efficiencies above about 95% were observed in all three cycles. The yields obtained in the first desorption cycle ranged from 3.19 to 48.49% depending on the resin type, the eluting agent and its concentration (Wołowicz 2019). In the present study, the sorption efficiency for the initial Zn (II) concentration of 1200 mg/L (in 3 M HCl) was in the range of 29—34% and the first cycle desorption efficiency was 65%.
In this study, the maximum sorption capacity for the sorption of zinc chloride complexes was obtained as 46.52 mg/g. The comparison of the sorption capacities of some anionic resins used as sorbent for the sorption of zinc chloride complex ions from HCl-based solutions is presented in Table 5. As PFA400MB anionic resin has a relatively high regeneration and reuse potential, as well as a higher sorption capacity most of the anionic resins presented in the table, it can be considered as a potential sorbent for the removal/recovery of zinc chloride complex ions in HCl-based wastewaters.
Conclusion
Although there are some studies on testing the performance of anion exchange resins for the recovery/removal of heavy metals from chlorinated strong acidic solutions, it is still seen as an area open to research.
In this study, it has been shown that Purolite A400MBOH and PFA400MB anion exchange resins can be used as sorbents for the sorption of zinc chloride complex ions from HCl-based solutions. But also, it has seen that the PFA400MB resin outperformed the A400MBOH resin.
In a strongly acidic medium acidified with HCl, zinc chloride complex ions can exist as fractions of species ZnCl4−2, ZnCl3− and ZnCl20. The SEM micrographs and also the EDAX spectra and elemental analyses confirm that zinc chloride complex ions bind to the resin surfaces.
Both Taguchi optimization and ANOVA were found to be effective tools to study the effects of process parameters (resin type, dosage, initial Zn(II) ion concentration, process temperature and process time, and agitation rate) on the ionic sorption of zinc chloride complex ions by anionic resins. Under optimum operating conditions, the PFA400MB resin shows the sorption capacity of 46.52 mg/g for removal of zinc chloride complexes. In the qe response, it was observed that all of factors investigated in the orthogonal array were statistically significant at the 95% confidence interval.
Microsoft Solver add-in in Excel has been successfully applied for nonlinear solutions of two or more parameter equilibrium isotherm models as well as kinetic models. The Langmuir model had a higher fit than the other six isotherm models examined and also the general order kinetic model was the kinetic model that best explained the sorption behaviour of zinc chloride complex ions, with reaction rates greater than 1.99 at all temperatures. Therefore, the sorption of zinc chloride complex ions onto the PFA400MB resin may be a chemisorption.
According to the results of tests for sorption and desorption of zinc chloride complex ions from HCl-based solutions, PFA400MB resin may be a convenient alternative for the removal/recovery of zinc chloride complex ions from chlorinated strong acidic environments. Based on the data obtained from this study, column tests can be carried out for the absorption of zinc chloride complex ions of PFA400MB resin from HCl-based solutions. Thus, possible industrial applications of the process can be evaluated in terms of economic and applicability parameters.
Data availability statement
All data are included in the paper or its Supplementary File.
Abbreviations
- a :
-
Initial sorption rate, mg/g min
- b :
-
Elovich constant, g/mg
- C 0 :
-
Initial concentrations of zinc ions in acidic solutions, mg/L
- C e :
-
Equilibrium concentrations of zinc ions at batch process conditions, mg/L
- C t :
-
Zinc concentrations in acidic solutions at time, mg/L
- h :
-
Initial sorption rate, mg/g min
- \({K}_{1,FS}\) :
-
Fritz-Schlunder equilibrium constant
- \({K}_{2,FS}\) :
-
Fritz-Schlunder equilibrium constant
- \({K}_{B}\) :
-
Baudu equilibrium constant
- \({K}_{F}\) :
-
Freundlich equilibrium constant, mg1−c Lc /g (c:1/nF)
- \({K}_{L}\) :
-
Langmuir equilibrium constant, L/g
- \({K}_{RP}:\) :
-
Redlich–Peterson equilibrium constant, L/g
- \({K}_{S}\) :
-
Sips equilibrium constant, (L/mg) ns
- k 1 :
-
Pseudo—First order rate constant, min−1
- \({k}_{2}\) :
-
Pseudo—Second order rate constant, g/mg min
- \({k}_{n}\) :
-
General order model rate constant, min / (g·mg) n−1
- m :
-
Mass of the resin, g
- \({m}_{1,FS}\) :
-
Fritz-Schlunder model exponent
- \({m}_{2,FS}\) :
-
Fritz-Schlunder model exponent
- N :
-
Total number of tests for Taguchi experimental design
- n :
-
General order model exponent
- n F :
-
Heterogeneity factor from Freundlich isotherm
- n S :
-
Sips model exponent
- q e :
-
Sorption capacity at equilibrium, mg/g
- q e, exp :
-
Sorption capacity measured experimentally
- q e,calc :
-
Sorption capacity predicted by the fitted model
- q e,mean :
-
Average of sorption capacity experimentally measured
- q e,PFO :
-
Theoretical sorption capacity from PFO, mg/g
- q e,PSO :
-
ThFritz-Schlunder isotherm saturation capacity, mg/georetical sorption capacity from PSO, mg/g
- q e,n :
-
Theoretical sorption capacity from general order model, mg/g
- q m,FS :
-
Fritz-Schlunder isotherm saturation capacity, mg/g
- q m,B :
-
Baudu isotherm saturation capacity, mg/g
- q m,L :
-
Langmuir constant for monolayer capacity, mg/g
- q m,S :
-
Sips isotherm saturation capacity, mg/g
- q t :
-
Sorption capacity at time, mg/g
- R :
-
Universal gas constant, 8.314 J / mol·K
- R 2 :
-
Coefficient of determination
- R adj 2 :
-
Adjusted determination factor
- T :
-
Temperature, °C
- t :
-
Sorption time, min
- V :
-
The aqueous volume of solution, L
- x :
-
Baudu isotherm model exponent
- y :
-
Baudu isotherm model exponent
- \({\alpha }_{RP}\) :
-
Redlich–Peterson constant, (1/mg) βRP
- \({\beta }_{RP}\) :
-
Redlich–Peterson exponent
References
Abidli A, Huang Y, Rejeb ZB, Zaoui A, Park CB (2022) Sustainable and efficient technologies for removal and recovery of toxic and valuable metals from wastewater: recent progress, challenges, and future perspectives. Chemosphere 292:133102. https://doi.org/10.1016/j.chemosphere.2021.133102
Ali I, Kon’kova T, Belkina I, Galunin E, Rysev A, Morozov A, Almalki ASA, Obaid RJ, Alsharif MA, (2021) Facile synthesis and characterization of advanced cobalt materials for degradative and adsorptive removal of carmoisine in water. IJEST 18:3221–3236. https://doi.org/10.1007/s13762-021-03529-2
Arias F, Sen TK (2009) Removal of zinc metal ion (Zn2+) from its aqueous solution by kaolin clay mineral: A kinetic and equilibrium study. Colloids Surf 348(1–3):100–108. https://doi.org/10.1016/j.colsurfa.2009.06.036
Bilal M, Ihsanullah I, Younas M, Shah M (2021) Recent advances in applications of low-cost adsorbents for the removal of heavy metals from water: a critical review. Sep Purif Technol 278:119510. https://doi.org/10.1016/j.seppur.2021.119510
Dakhem M, Ghanati F, Mohammadian MA, Sharif M (2022) Tea leaves, efficient biosorbent for removal of Al3+ from drinking water. IJEST 19:10985–10998. https://doi.org/10.1007/s13762-022-04313-6
Davis R, John P (2018) Statistical approaches with emphasis on design of experiments applied to chemical processes. InTechOpen. https://doi.org/10.5772/65616
Duan C, Ma T, Wang J, Zhou Y (2020) Removal of heavy metals from aqueous solution using carbon-based adsorbents: a review. JWPE 37:101339. https://doi.org/10.1016/j.jwpe.2020.101339
Fernández-López JA, Angosto JM, Roca MJ, Miñarro MD (2019) Taguchi design-based enhancement of heavy metals bioremoval by agroindustrial waste biomass from artichoke. Sci Total Environ 653:55–63. https://doi.org/10.1016/j.scitotenv.2018.10.343
Foo KY, Hameed BH (2010) Insights into the modeling of adsorption isotherm systems. J Chem Eng 156:2–10. https://doi.org/10.1016/j.cej.2009.09.013
Gîlcă E, Măicăneanu A, Ilea P (2014) Removal of zinc ions as zinc chloride complexes from strongly acidic aqueous solutions by ionic exchange. Cent Eur J Chem 12(8):821–828. https://doi.org/10.2478/s11532-014-0504-8
Gîlcă E, Măicăneanu A, Imre-Lucaci Á, Ilea P (2017) ZnCl4-2 sorption on amberlite IRA410 resin using Taguchi’s methodology for design of experiments. Chem Eng Commun 204(3):382–387. https://doi.org/10.1080/00986445.2016.1205980
Googerdchian F, Moheb A, Emadi R, Asgari M (2018) Optimization of Pb(II) ions adsorption on nanohydroxyapatite adsorbents by applying Taguchi method. J Hazard Mater 349:186–194. https://doi.org/10.1016/j.jhazmat.2018.01.056
Ho YS, McKay G (1998) Kinetic models for the sorption of dye from aqueous solution by wood. Trans IChemE 76:183–191. https://doi.org/10.1205/095758298529326
Ho YS, McKay G (1999) The sorption of lead(II) ions on peat. Wat Res 33(2):578–584. https://doi.org/10.1016/S0043-1354(98)00207-3
Horne RA, Holm RH and Meyers MD (1957) The adsorption of zinc(II) on anion-exchange resins. II. Stoichiometry, thermodynamics, loading studies, Dowex-2 adsorption and factors influencing the rate of the adsorption process. J Phys Chem 61(12):1655–1661
Hu S, Xu C, Srinivasakannan C, Tan X, Ni S, Zhang J, Li X, Zhang H, Li S (2022) Recovery of zinc and iron from hot-dip galvanizing spent pickle liquor using solvent extraction. J Mol Liq 362:119741. https://doi.org/10.1016/j.molliq.2022.119741
Inès M, Mekki S and Ghribi D (2022) Treatment of heavy metals contaminated water: use of B. mojavensis BI2 derived lipopeptide and palm waste flour. Water Sci Technol 86(5):1083–1094. https://doi.org/10.2166/wst.2022.247
Korake SR, Jadhao PD (2021) Investigation of Taguchi optimization, equilibrium isotherms, and kinetic modeling for cadmium adsorption onto deposited silt. Heliyon 7:e05755. https://doi.org/10.1016/j.heliyon.2020.e05755
Kononova ON, Mikhaylova NV, Melnikov AM, Kononov YS (2011) Ion exchange recovery of zinc from chloride and chloride–sulfate solutions. Desalination 274:150–155. https://doi.org/10.1016/j.desal.2011.02.005
McMahon ME, Santucci RJ, Scully JR (2019) Advanced chemical stability diagrams to predict the formation of complex zinc compounds in a chloride environment. RSC Adv 9:19905–19916. https://doi.org/10.1039/C9RA00228F
Miesiac I (2005) Removal of Zinc(II) and Iron(II) from Spent Hydrochloric Acid by Means of Anionic Resins. Ind Eng Chem Res 44:1004–1011. https://doi.org/10.1021/ie0493762
Mohan S, Kumar V, Singh DK, Hasan SH (2017) Effective removal of lead ions using graphene oxide-MgO nanohybrid from aqueous solution: isotherm, kinetic and thermodynamic modelling of adsorption. J Environ Chem Eng 5:2259–2273. https://doi.org/10.1016/j.jece.2017.03.031
Peng W, Li H, Liu Y, Song S (2017) A review on heavy metal ions adsorption from water by graphene oxide and its composites. J Mol Liq 230:496–504. https://doi.org/10.1016/j.molliq.2017.01.064
Pietrelli L, Ferro S, Vocciante M (2018) Raw materials recovery from spent hydrochloric acid-based galvanizing wastewater. J Chem Eng 341:539–546. https://doi.org/10.1016/j.cej.2018.02.041
Purolite (2023) Product Data Sheet. https://www.purolite.com/product-pdf/A400MBOH.pdf Accessed 03 June 2023
Purolite (2023) Product Data Sheet. https://www.purolite.com/product-pdf/PFA400MB.pdf Accessed 03 June 2023
Saadi R, Saadi Z, Fazaeli R, Fard NE (2015) Monolayer and multilayer adsorption isotherm models for sorption from aqueous media. Korean J Chem Eng 32(15):787–799. https://doi.org/10.1007/s11814-015-0053-7
Sinha MK, Sahu SK, Meshram P, Pandey BD (2014) Solvent extraction and separation of zinc and iron from spent pickle liquor. Hydrometallurgy 147–148:103–111. https://doi.org/10.1016/j.hydromet.2014.05.006
Taguchi G (1986) Introduction to Quality Engineering: Designing Quality into Products and Processes. Asian Productivity Organization, Tokyo
Tang J, Li Y, Wang X, Daroch M (2017) Effective adsorption of aqueous Pb2+ by dried biomass of Landoltia punctata and Spirodela polyrhiza. J Clean Prod 145:25–34. https://doi.org/10.1016/j.jclepro.2017.01.038
Vergili I, Soltobaeva G, Kaya Y, Gönder ZB, Çavuş S, Gürdağ G (2013) Study of the removal of Pb(II) using a weak acidic cation resin: kinetics, thermodynamics, equilibrium, and breakthrough curves. Ind Eng Chem Res 52:9227–9238. https://doi.org/10.1021/ie400630d
Wagner AS, Eder LC, Royer B, dos Santos BD, Tatiana C, da Silva EA, Claudio AN (2012) Application of aqai stalks as biosorbents for the removal of the dye procion blue MX-R from aqueous solution. Sep Sci Technol 47:513–526. https://doi.org/10.1080/01496395.2011.616568
Wang J, Guo X (2020) Adsorption kinetic models: physical meanings, applications, and solving methods. J Hazard Mater 390:122156. https://doi.org/10.1016/j.jhazmat.2020.122156
Wasewar KL, Atif M, Prasad B, Mishra IM (2008) Adsorption of zinc using tea factory waste: kinetics, equilibrium and thermodynamics. Clean: Soil, Air, Water 36:320–329. https://doi.org/10.1002/clen.200700139
Wołowicz A (2019) Zinc(II) removal from model chloride and chloride–nitrate(V) solutions using various sorbents. Physicochem Probl Miner Process 55(6):1517–1534. https://doi.org/10.5277/ppmp19080
Wołowicz A, Hubicki Z (2016) Sorption behavior of dowex PSR-2 and dowex PSR-3 resins of different structures for metal (II) removal. Solvent Extr 34(4):375–397. https://doi.org/10.1080/07366299.2016.1187983
Xinyu C, Hossain MF, Duan C, Lu J, Tsang YF, Islam MS, Zhou Y (2022) Isotherm models for adsorption of heavy metals from water—a review. Chemosphere 307:135545. https://doi.org/10.1016/j.chemosphere.2022.135545
Zhou K, Wu Y, Zhang X, Peng C, Cheng Y, Chen W (2019) Removal of Zn (II) from manganese-zinc chloride waste liquor using ion exchange with D201 resin. Hydrometallurgy 190:105171. https://doi.org/10.1016/j.hidromet.2019.105171
Zolfaghari G, Esmaili-Sari A, Anbia M, Younesi H, Ghasemian MB (2013) A zinc oxide-coated nanoporous carbon adsorbent for lead removal from water: optimization, equilibrium modelling, and kinetics studies. IJEST 10:325–340. https://doi.org/10.1007/s13762-012-0135-6
Acknowledgements
I would like to thank Vedat Şimşek, Purolite Turkey Office representative, who supported the supply of resins.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). The research has no financial support affiliation.
Author information
Authors and Affiliations
Contributions
All experimental and modelling parts of this study were planned and carried out by Necla BARLIK. The study was prepared as a manuscript by her to be submitted to the International Journal of Environmental Science and Technology.
Corresponding author
Ethics declarations
Conflict of interest
The author declares there is no conflict.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Editorial responsibility: Maryam Shabani.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Barlık, N. Sorption of zinc chloride complexes from acid-based solutions by using anionic resins: process optimization and modelling. Int. J. Environ. Sci. Technol. (2024). https://doi.org/10.1007/s13762-024-05829-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13762-024-05829-9