Abstract
A key aspect of climate change is its impact on river water quality. Most research focuses on the impact of physiochemical parameters on water quality. However, the long-term shifts in temperatures and weather patterns coupled with anthropogenic activities play a significant role in river contamination. Metals are known to have toxic effects and environmental persistence. This study evaluated the heavy metal content of the Kaap River, where mining and agriculture are the primary land use. A 7-year study was conducted to investigate the seasonal relationship between heavy metals and physiochemical parameters (EC and pH). Fe, Al, As, and Mn concentrations were analysed in a laboratory accredited by the South African National Accreditation System (SANAS). The Python package Seaborn was used to generate heat maps for improved data visualization. Seasonal and temporal fluctuations had a combinatorial impact on Mn, Fe, and Al levels of the river. However, As levels were unaffected. The pH of the rivers was within the recommended range, despite flow regime, seasonal, and time-dependent fluctuations. Seasonal and temporal variations were also observed for EC, with the highest value of 42.35 mS/m being recorded during the winter of 2022, exceeding the recommended threshold of 30 mS/m. The correlation analysis revealed positive and significant correlations for the EC/pH and Al/Fe combinations and a weak degree of association for other parameters (P < 0.05). A permanent monitoring of water quality is required to ensure sustainable livelihoods and the safety of Kaap River water, which is subject to significant heavy metal fluctuations over time.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Climate change is anticipated to increase the frequency of extreme weather events, which will have direct and indirect effects on the dynamics of contaminants in aquatic ecosystems. Changes in the physical and geochemical properties of the soil–contaminant water system are one example. Several authors have investigated the influence of climate change on river water quality (Fan and Shibata 2015; Xia et al. 2015; Rostami et al. 2018). Some of these studies have examined the impact of temperature on water quality, while others have investigated the effects of surface runoff. These studies suggest that climate change has both direct and indirect effects on the quality of surface water. For instance, as a direct result of climate change, the rising temperatures have caused deteriorations in quality freshwater ecological status. Climate change may increase the risk of flooding, which increases the likelihood that contaminants will be remobilized in floodwater and that contaminated sediment and water will reach freshwater and marine environments (Ahmed et al. 2020). The qualitative effects of climate change on heavy metal contamination in marine ecosystems have been discussed (Schiedek et al. 2007). As a dynamic component of the interaction between the lithosphere, biosphere, and hydrosphere, dissolved organic matter (DOM) has the potential to affect the global carbon cycle and climate change (Marín-Spiotta et al. 2014; Pagano et al. 2014). As a crucial redox-active transition metal, iron (Fe) can regulate the geochemical cycle of trace elements (Bazzani et al. 2023; Sunda 2012). Moreover, it is a key nutrient in the ocean system that inhibits primary productivity in many ocean regions and exacerbates issues caused by cyanobacterial blooms (Sander et al. 2015).
Erosion and deposition of rocks and soils are the sources of heavy metals. Anthropogenic sources are another potential origin, such as discharges and pollutants from industry and urbanization (Benamar et al. 1999). When heavy metal concentrations in the river exceed normal levels, they can have a significant negative impact on the ecosystem due to their toxicity to microbes, plants, animals, and mankind (Keshav Krishna et al. 2011). As a result, understanding heavy metal content and distribution in sediments is critical. Knowing the temporal variability and controls on manganese (Mn) concentrations in runoff from upland catchments enables the prediction of undesirable Mn concentrations in water supplies and the identification of water supply catchments at risk from elevated Mn levels. Although Mn is abundant in the environment, it has rarely been the subject of in-depth hydrological studies in catchments located in mountainous regions. However, the accumulation of heavy metals in the food chain is worrisome due to its potential long-term impacts on human health (Raghunath et al. 1999; Li et al. 2004). Fe is predominantly present in water as either ferrous iron, which is soluble, or ferric iron, which is insoluble. Water containing ferrous iron is transparent and colourless due to the iron’s complete dissolution. Heavy metals like aluminium (Al) are toxic to living things and serve no biological function (Siddiquee et al. 2015; Turpeinen et al. 2004). Al toxicity is significantly affected by pH (Jaishankar et al. 2014), with acid rain-induced low pH enhancing its toxicity (Gardner and Al-Hamdani 1997). On the other hand, Al mining and processing raises its concentration in the environment (Agency for Toxic Substances and Disease Registry 2008). It has also been found to cause several illnesses in people, animals, and plants (Barabasz et al. 2002). Arsenic (As), due to its semimetallic characteristic, toxicity, and carcinogenic qualities, creates both environmental and health concerns (Hughes et al. 1988; Singh et al. 2007). As is primarily produced through pest management, mining, and manufacturing processes (Bundschuh et al. 2011). About 130 million people around the world are exposed to As concentrations above 50 g/L (WHO 2021), which is higher than the recommended level of 10 g/L (Fatoki et al. 2013). Furthermore, electrical conductivity (EC) and pH can classify rivers (Rim-Rukeh et al. 2006). The percentage of dissolved particles in water determines water’s electrical conductivity (Anna 2018–124), whereas pH affects the toxicity (Gardner and Al-Hamdani 1997) and bioavailability of heavy metals (Adokpayi et al. 2014).
Several studies that have been carried out across the globe have focused primarily on evaluations of the concentration of heavy metals (Muhammad 2023), heavy metal and metalloid contamination in the sediments (Shafiq et al. 2011; Saleem et al. 2019; Addo-Bediako et al. 2021), impact of heavy metals on aquatic ecosystem (Wei et al. 2022; Rani et al. 2022; Afzaal et al. 2022; Inayat et al. 2023). Extensive literature about the accumulation of heavy metals has been widely reported in countries such as in Pakistan, United states of America (USA), Brazil, Italy, and Bangladesh (Ajima et al. 2015; Pal and Maiti 2018; Saleem et al. 2019; Gevorgyan et al.2021; Lipy et al. 2021). It is important to acknowledge that metals do not possess intrinsic toxic properties. However, when metals are combined, especially in aqueous environments, they present a considerable problem and are consistently detrimental in such circumstances. Metals may be classified into four distinct categories according to their inherent features as such this current study focused on metals essential for metabolic processes. Throughout the literature search of this study, it was noted that few studies have looked at the long-term seasonal variation of heavy metals in rivers, mostly look at a period of two to three years particularly in the global south. It was further noted that within the Sub-Saharan region, there is limited literature that seeks to assess the interaction between temporal and seasonal distribution of heavy metals and physiochemical properties. Therefore, this study was carried over a period of seven years from 2016 to 2022 at three strategic points, downstream, midstream, and upstream, of the confluence of Crocodile River and Kaap River.
The primary objective of the present study, which is the first exhaustive investigation of the distribution of heavy metals in the Kaap River, is to evaluate the seasonal impact of heavy metal accumulation/contamination over a seven-year period. This is a crucial study because the Kaap River is classified as Class C, which is primarily used for agricultural and public supply purposes. Consequently, Africa is one of the largest continents, afflicted by numerous environmental issues, such as water contamination and biodiversity loss (Fernandez-Luqueno et al. 2013). The research also linked the physiochemical characteristics (pH and EC) of the river with the occurrences of heavy metals. This study also seeks to determine whether or not long-term changes in average temperatures and weather patterns that affect micro- and macroclimates on earth, in conjunction with human activities, play a significant role in the formation/accumulation of metal contaminants in rivers.
Materials and methods
Study area
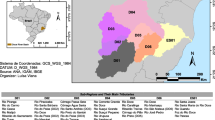
Kaapmuiden is a small farming area located approximately 40 km east of Mbombela (formerly known as Nelspruit) at the confluence of the Kaap River and the Crocodile River in Mpumalanga, South Africa. The region has a subtropical climate and fertile farmlands where sugarcane and other subtropical fruits and vegetables are grown. The Kaap River (Fig. 1) is one of the largest tributaries of the Crocodile River system, with a catchment area of 1640 km2. It flows and drains through areas dominated by mining (active mines, defunct mines, and decanting mines) and agricultural activities before joining the Crocodile River at Kaapmuiden. Crocodile River water quality is generally characterized by high metal contamination, particularly Mn and As, as well as high sulphates resulting from mining activities in the Kaap River sub-catchment. The sub-catchment plays a pivotal role in the region primarily because it fosters socio-economic development and is a part of the internationally significant water resources flowing into Mozambique.
Data collection
To determine the influence of seasonal and temporal variations on heavy metals and physiochemical parameters, three sampling stations were randomly selected from the Kaap River (see Fig. 1). Sampling was conducted between 2016 and 2022 at three strategic points, downstream, midstream, and upstream, of the confluence of Crocodile River and Kaap River using the grab sampling technique. Before treatment of the samples, pH and EC (µs/cm) were measured using a Hach multi-probe metre which was calibrated using buffers before use, potentiometric analytical method was followed for analysing both parameters. For sample collection, polyethylene bottles were used, and sampling dates and times were marked with a marker. To minimize precipitation and adsorption on container walls, samples were prepared by adding ultra-pure nitric acid to reduce the pH of the sample to less than 2.0.
Analysis of parameters
Fe, Al, Mn, and As were analysed using an atomic absorption spectrophotometer. The analytical method is based on the Beer Lambert’s law which states that absorbance of light emitted by a light to a detector is directly proportional to the concentration. A sample is aspired into a flame, enabling the sample element to be changed into an atomic vapour of that element that is analysed, absorbing the radiation of specific wavelength produced by a light source (hollow cathode lamp) of that specific metal. The wavelength of radiation given off by the light source is similar as that of absorbed by the atoms in the flame, thus determining the concentration of the metal in the solution.
Statistical data analysis
Since this current study dealt with large dataset, the Seaborn software was used to do the statistical analysis. The Seaborn uses a python which is a popular programming language that offers numerous data analysis and visualization tools and libraries. Water quality datasets were analysed using Seaborn (Python), including plotting and analysing the distribution and correlation of variables with Matplotlib libraries. The Python package Seaborn was used to generate the required heat maps for improved data visualization. For correlation studies, the correlations significance and confidence intervals were calculated.
Results and discussion
In-depth study of the Fe and Mn concentrations in the Kaap River
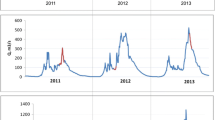
The concentration of Fe (0.90 mg/L) in the river water was considerably (p < 0.005) higher in Autumn of 2021 compared to other years and seasons (Fig. 2). The second greatest Fe concentration (0.64 mg/L) was recorded during the same season (Autumn) in 2017, suggesting that there may be a potential anthropogenic source of Fe during the Autumn season. It is also plausible that significant contamination was caused by diminished river water flow during the Autumn. The values observed here are much greater than the 0.3 mg/L threshold suggested by the World Health Organization (WHO 2021), and may pose a health risk to the neighbouring communities. On the other hand, significantly reduced Fe concentrations were observed throughout the Spring season across all years (2016–2022).
Arain et al. (2009) tested heavy metal ions in Indus River water from the Kotri and Ghulam Muhammad dam for one year. They discovered Fe and Mn concentrations that exceeded WHO safety standards. Summer’s peak water volumes decreased metal emissions, while winter’s precipitation decreased contamination. Very high Fe concentrations were discovered and according to their assessment, sources of heavy metals include domestic discharge, industrial waste, and mining activities. In a different but related study (Reza and Singh 2010), water samples were collected from twelve various locations along the river during the summer and winter seasons to demonstrate the seasonal variations in the river’s water quality in relation to heavy metal contamination. The highest concentrations of Fe were discovered to be 64,6 and 95,0 g/L during the summer and winter, respectively. The authors acknowledged that these variations could also be attributed to the soil–water interaction, particularly in the winter months.
When compared to subsequent years, the average concentration of Mn in the river water was exceptionally high in 2016 (Fig. 2). These 2016 observations and seasonal phenomenon may also be attributable to the significantly decreased precipitation observed during El Nio (drier seasons and drought conditions) in South Africa (Gqomfa et al. 2023), resulting in elevated heavy metal concentrations due to extremely limited water flow. Moreover, the most notable and consistent Mn average levels over the years have been 0.01 mg/L, this trend was conspicuous because Mn is a prevalent element in the earth’s crust and is found in a variety of minerals in rocks and sediments (Essington 2015). However, given that studies have shown that Mn in water at a concentration of 0.1 mg/L can have detrimental impacts on children’s health (Ying et al. 2017), these consistent values (0.01 mg/L) are equally problematic and necessitate government and community intervention. In spite of the fact that the present study did not measure precipitation over the course of the study, it cannot draw any significant conclusions about the variation of heavy metal concentrations based on variations in the flow rate of the river throughout the study.
Additionally, the winter of 2021 saw an alarmingly rapid increase in Mn concentrations of 0.05 mg/L. Kaap River is one of the major tributaries of the Crocodile River system, with a catchment area dominated by mining activities (active mines, defunct mines, and decanting mines) and intensive agricultural activities, which may have contributed to Mn contamination in the river. This observation may not come as a surprise, given that winter typically experiences little precipitation, which may contribute to the concentration of undiluted heavy metals (e.g. Mn) originating from anthropogenic sources. The World Health Organization considers Mn concentrations of 0.05 mg/L to be significantly higher, so this poses severe safety concerns (WHO 2021). Elsewhere, (Khallaf et al. 2021) demonstrated that winter Mn concentrations are dependent on the season and are typically higher during the winter. They examined the distribution of Mn in the sediments of the Bahr Shebeen Nilotic Canal (BSC) from September 2014 to December 2015 at various sites. The concentrations of Mn followed the pattern of winter > autumn > spring > summer. Siraj et al. (2016) also discovered heavy metal contamination in the waters of the River Kabul in Pakistan. The range of heavy metal concentrations (μg/L) found was Mn 16.7–38.5. Sources of heavy metals discharged effluents from sewage, industrial sources, and agricultural runoff. They also determined that Mn concentrations exceeded biologically permissible limits.
Seasonal changes impact heavy metal concentrations; however, the rapid development of mining and agriculture in South Africa and the resulting rise in the demand for farm irrigation are the primary causes of the river metal contamination crisis, with far-reaching consequences for public health (FAO 2007; Wright and Welbourne 2002). An analysis conducted by Saleem et al. (2019) has found that there is a significant difference in the Zn and Mn concentrations in the River Jhelum at Muzaffargarh when compared with both the US EPA and World Health Organization standards for drinking water. Moreover, the study found that under the low flow season, iron (Fe) concentrations were extremely high and posed a potential risk to human health (Saleem et al. 2019).
Comprehensive analysis of Al and As levels in the Kaap River
The seven-year study period made it possible to comprehend seasonal variations in Al and As concentrations. Al and As concentrations (Fig. 3) in the Kaap River were assessed because controlling water quality requires monitoring its chemical composition and identifying the presence of contaminants (Brindha et al. 2020).
The concentrations of Al varied significantly during the course of the seasons and the time period (2016–2022). For all seasons in 2021, Al concentrations were comparatively and significantly higher. All the tested samples during the seven years of study (2016–2022) have typically always contained traces of Al due to the abundance of Al in the Earth’s crust (Korium et al. 2006).
However, the 2017 (0.59 mg/L) and 2021 (1.10 mg/L) autumn samples had the highest Al concentrations. These winter observations may be attributed to domestic discharge and agricultural effluent (Wu et al. 2008). Other factors that may have contributed to elevated Al levels in the river is contamination from nearby mining activity, the decomposition of organic materials, agricultural waste, fertilizers, and pesticides. These concentrations exceeded threefold the World Health Organization-recommended safe consumption limit of 0.2 mg/L (WHO 2021), which has the potential to cause Alzheimer’s disease, brain damage, and other health complications in humans (Edokpayi et al. 2014; Afzaal et al. 2022). A different study Senze et al. 2021a conducted on sediments collected from the Nysa Szalona, Strzegomka, and Bystrzyca rivers (Poland) also found that autumn sediments contain more Al than spring sediments.
The summer of 2016 (0.26 mg/L/), 2019 (0.23 mg/L/), 2020 (0.23 mg/L/), 2021 (0.23 mg/L/), and 2022 (0.44 mg/L/) also showed slightly higher Al concentrations, which coincided with rainy weather because the summer months in most parts of South Africa are characterized by precipitation or rain. Prior research has shown that precipitation from the atmosphere is the primary source of metal contamination in surface waters (Wong et al. 2003; Wu et al. 2008), and thus, these moderately elevated Al concentrations can be attributed to the rainy seasons in summer.
Heavy metal contamination is severe in South African rivers, and environmental intervention is evident. Jackson et al. (2009) reported significantly higher Al concentrations in the Plankenburg (13.6 mg/l) and Diep rivers (4 mg/L). These levels were considerably higher than the Department of Water Affairs and Forestry’s recommended water quality threshold.
Senze et al. 2021b conducted a four-year study to gain a comprehensive understanding of river Al concentrations. Al concentrations in the water of the Nysa Szalona and Strzegomka rivers increased during spring and autumn. In the autumn, however, the Nysa and its tributaries had slightly higher levels, implying a larger influence from agriculture and runoff. The authors asserted that spring river waters in Strzegomka and Bystrzyca convey Al-rich post-winter thaws, most likely due to intense surface discharge and terrain slope.
The amount of As in the Kaap Rivers was also examined as part of this study, along with its seasonal effects throughout a seven-year cycle. Despite mining and agricultural activities taking place close to the Kaap River, measurements of the As levels in the water showed that they were within the permitted range of 0.01 mg/L in the samples (see Fig. 3). Due to its effect on human health, As is becoming a global problem, and a great deal of research has been conducted worldwide to ascertain the level of contamination in water (Fatoki et al. 2013); therefore, there is a need for extensive monitoring of As contamination in Africa as well. According to Ahoulé et al. 2015, As concentrations in groundwater and surface water in Africa are exceedingly high. For example, 566 g/L of As was detected in the surface water of Ethiopia (Rango et al. 2013) and up to 8250 g/L in the surface waters of Ghana (Serfor-Armah et al. 2006), both of which are attributed to human activities (Kusimi and Kusimi 2012). However, As mitigation activities cannot be effectively implemented or maintained without the support of the local community (Tet Nay Tun 2003).
Comprehensive analysis of pH and EC in the Kaap River
Figure 4 shows the physicochemical parameters (pH and EC) in samples taken from the Kaap River over the course of a seven-year cycle in the winter, summer, autumn, and spring. The water samples’ average pH ranged from 7.67 (summer 2022) to 8.43 (winter 2021). The pH levels from 2016 to 2022 were within the legal range of 6.5–8.5 (WHO 2021) throughout the entire seven-year cycle and in all seasons. However, it was also evident that seasonal changes had significantly impacted (directly or indirectly) the water’s pH. According to the pH results, autumn river samples in the study area were slightly more acidic than samples collected in spring, winter, and summer. The pH levels of samples collected during the seasons of 2022 were also relatively lower (slightly acidic) compared to the other years. In addition, the winters of 2017 (pH = 8.26), 2019 (pH = 8.22), and 2021 (pH = 8.43) had the highest pH levels. However, pH values were also consistently higher in the spring and summer during the same time period. The observed increase in pH can contribute to the build-up of algal growth in the rivers (Edokpayi et al. 2014) and may lessen the toxicity of heavy metals (Aktar et al. 2010). In a separate study, Edokpayi et al. (2014) analysed the water quality of the Dzindi River in Limpopo Province (South Africa) and found that the pH of the samples varied between 7.47 and 7.53. According to the authors, maintaining the correct pH of the river water is crucial because pH can influence plant growth and the bioavailability of heavy metals. The presence of heavy metals in water is contingent upon the pH level of the water. The solubility of heavy metal ions in water is contingent upon the pH level of the solution (Mitra et al. 2022). Typically, heavy metal ions exhibit a high degree of solubility in aqueous systems when subjected to low pH conditions due to their presence in the cationic form (Hama Aziz et al. 2023). Nevertheless, as the pH level rises, specifically above a value of 7.00, the solubility of metal ions experiences a decline as a result of the creation of hydroxide complexes.
The range of EC concentration in the sampled water was 16.30 (autumn 2022) to 42.35 mS/m (winter 2022). The EC of both the spring and winter seasons between 2016 and 2022 was higher than the recommended threshold of 30 mS/m for water resources (Fig. 4). Given that measuring the EC of water provides an approximation of the quantity of multiple ions (such as carbonate, sulphate, sodium, magnesium, and so on), all of which bear an electrical charge (DWAF 1996), the existence of numerous ions is plausible in these samples. More research is therefore needed to confirm the presence of these ions in the Kaap River during the spring and winter, quantify their levels, and assess their impact on the ecosystem and human health.
Correlation studies: analysis of Seasonal and Temporal variabilty
Figure 5 shows the correlation heat maps and linear graphs for the relationship between heavy metals and the physiochemical characteristics (EC and pH) of the water.
The motivation for studying the relationship between pH and heavy metals stemmed from the fact that high acidity improves the solubility of certain chemical substances, raising their concentrations in the environment (Embrapa 2006). Aprile et al. (2005) discovered that the harmful effect of heavy metals is greatly reliant on environmental factors such as pH, among other things, and that long-term exposure to heavy metals may produce physiological changes (mutagenesis and carcinogenesis). EC is also useful in understanding the relationship between dissolved chemicals and their effect on water salinity. That is, pH and EC can help to understand the behaviour of heavy metals investigated here.
The concentrations of Al and Fe (r = 0.88) were strongly positively correlated (Fig. 5). The most significant association, though, was established throughout the summer and autumn seasons. It is possible that the high concentrations of Al and Fe in the river occurred at the same time for the same reason: both metals originated from the same anthropogenic source. Additionally, it is possible that the high concentrations of Al and Fe in the river were influenced by precipitations in the summer season that promote transference of the metals from the nearby farms and mines. According to Shafi et al. (2018), there has been no comparison of metal composition in diverse components of an urban aquaculture pond during multiple seasons and its location in the vicinity of a coal mining city. The enduring existence of heavy metals in the environment presents substantial hazards to both human well-being and ecological systems. The build-up of heavy metals in organisms resulting from water pollution by these metals has emerged as a significant issue, mostly owing to the potential damage they represent to human beings.
Due to the fluctuating concentrations of the heavy metals detected in the water samples over the course of the study, no additional direct correlations between heavy metals and physiochemical parameters (EC and pH) were found. This, however, contradicts some of the discoveries made by other environmental scientists. Elsewhere, a correlation has been discovered between the amount of Al in the sediment and the pH of both the sediment and the water (Senze et al. 2021a). This correlation has been found in a number of different environments. According to the researchers (Barabasz et al. 2002; Kabata-Pendias 2010), bottom sediments often absorb Al in the form of metastable compounds. Al is released into the water when the acidity of the water increases, to the point where Al concentrations of up to 5 mg dm3 can be attained. Another study discovered a link between pH, Mn, and Fe levels in borehole water (Moyosore et al. 2014). They identified an inverse correlation between Mn concentrations and pH in low density areas as well as an increase in Fe concentrations when the pH was lower in high density areas. These findings are not surprising given that the chemical activity of heavy metals like Fe is mediated by pH and redox potential (Khan et al. 2017). The concentrations of dissolved Fe and Mn in groundwater were also shown to be controlled by redox conditions and pH, with Fe and Mn becoming more soluble under acidic environments (Zhai et al. 2021). On the other hand, Fatoki et al. 2013 reported that, among heavy metalloids, As is particularly sensitive to mobilization (pH 6.5–8.5).
During the study period, there was a positive correlation between pH and EC of the water in all seasons. Exploring the link that exists between pH and conductivity was critical since the existence of any hydrogen ions influence the pH, as well as the levels of conductivity. The association between EC and pH in rivers is seen in Fig. 5. The correlation coefficient (r) was higher across all seasons, showing that pH may have had a major impact on river conductivity. There was, however, no positive correlation between EC and heavy metal concentrations. Recently, the relationship between EC and Fe 2+ was evaluated by Erebho (2022), who found that EC was strongly related to Fe2+ in the Ikpoba river but only moderately related in the Ogba river.
Conclusion
Autumn samples contained the highest concentrations of Al exceeding the World Health Organization’s safe consumption limit. Despite the presence of mining and agricultural activities near the Kaap River, As levels in the water were measured to be within the prescribed limit. In Autumn of 2021, the Fe concentration was substantially higher than the permissible limit, posing a health risk to the surrounding communities. The Mn concentrations followed the order of winter > autumn > spring > summer. In 2016, the average concentration of Mn in river water was exceptionally high, which could lead to adverse health effects necessitating urgent environmental intervention. Temporal and seasonal variations significantly affected physiochemical parameters. Strong and weak correlations between heavy metals and physiochemical parameters were established during the seven-year period of study. Overall, this study’s findings could be attributed to both anthropogenic activities and substantial contamination brought on by the seasonal changes in river flow.
Recommendations
It is recommended that the Kaap Rivers be routinely analysed for heavy metals, pH, and EC to determine the contamination risk and protect river-using community members. It is also advised that river sediment analysis be performed to ensure the safe levels. Given that heavy metals in water concentrations above the prescribed limit have negative effects on the environment and humans, a collaborative effort between business, government, and the community is imminent to remedy the situation in the Kaap River. The proliferation of heavy metal ions, which pose significant risks to human health, is a direct consequence of contemporary industrial mining, agricultural practices, and several other anthropogenic activities. Therefore, the effective eradication of a particular phenomenon is of utmost importance for both the well-being of human beings and the preservation of the natural environment in which they inhabit. Further investigation is required in order to fully comprehend the mechanisms and principles behind the degradation of heavy metal complexes and the subsequent recovery of heavy metal ions via the implementation of advanced oxidation processes (AOPs).
Data availability
Information/data supporting the results of this study can be obtained from the corresponding author, upon request.
References
Addo-Bediako A, Nukeri S, Kekana M (2021) Heavy metal and metalloid contamination in the sediments of the Spekboom River. South Africa Appl Water Sci 11:133. https://doi.org/10.1007/s13201-021-01464-8
Afzaal M, Hameed S, Liaqat I et al (2022) Heavy metals contamination in water, sediments and fish of freshwater ecosystems in Pakistan. Water Pract Technol 17:1253–1272. https://doi.org/10.2166/wpt.2022.039
Agency for Toxic Substances and Disease Registry (2008). Public health statement aluminium. ATSDR Publication CAS#7429-90-5
Ahmed J, Wong LP, Chua YP et al (2020) Quantitative microbial risk assessment of drinking water quality to predict the risk of waterborne diseases in primary-school children. IJERPH 17:2774. https://doi.org/10.3390/ijerph17082774
Ahoulé DG, Lalanne F, Mendret J et al (2015) Arsenic in African waters: a review. Water Air Soil Pollut 226:1–13. https://doi.org/10.1007/s11270-015-2558-4
Ajima MNO, Nnodi PC, Ogo OA, Adaka GS, Osuigwe DI, Njoku DC (2015) Bioaccumulation of heavy metals in Mbaa River and the impact on aquatic ecosystem. Environ Monit Assess 187:768. https://doi.org/10.1007/s10661-015-4937-0
Aktar MD, Paramasivam M, Ganguly M et al (2010) Assessment and occurrence of various heavy metals in surface water of Ganga river around Kolkata: a study for toxicity and ecological impact. Environ Monit Assess 160:207–213. https://doi.org/10.1007/s10661-008-0688-5
Anna FR (2018) IOP conference series. Environ Earth Sci. vol 118, p 012019
Aprile FM, Siqueira GW, Parente AH (2005) Occurrence and potentially toxic of heavy metals in aquatic ecosystems and their effects on organisms health. Rev Tecnol 1(2):40–47
Arain MA, Wattoo FH, Sarwar Wattoo MH et al (2009) Simultaneous determination of metal ions as complexes of pentamethylene dithiocarbamate in Indus river water, Pakistan Arab. J Chem 2:25–29. https://doi.org/10.1016/j.arabjc.2009.07.007
Barabasz W, Albinska D, Jaskowska M, Lipiec J (2002) Ecotoxicology of aluminium. Pol J Environ Stud 11:199–203
Bazzani E, Lauritano C, Saggiomo M (2023) Southern ocean iron limitation of primary production between past knowledge and future projections. J Mar Sci Eng 11:272. https://doi.org/10.3390/jmse11020272
Benamar MA, Toumert I, Tobbeche S et al (1999) Assessment of the state of pollution by heavy metals in the surficial sediments of Algiers Bay. Appl Radiat Isot 50:975–980. https://doi.org/10.1016/S0969-8043(98)00111-0
Brindha K, Paul R, Walter J et al (2020) Trace metals contamination in groundwater and implications on human health: comprehensive assessment using hydrogeochemical and geostatistical methods. Environ Geochem Health 42:3819–3839. https://doi.org/10.1007/s10653-020-00637-9
Bundschuh J, Bhattacharya P, Sracek O et al (2011) Arsenic removal from groundwater of the Chaco-Pampean Plain (Argentina) using natural geological materials as adsorbents. J Environ Sci Health Part A 46:1297–1310. https://doi.org/10.1080/10934529.2011.598838
DWAF (1996) South African water quality guidelines: aquatic ecosystems (2nd edn), vol 7, Pretoria, South Africa
Edokpayi JN, Odiyo JO, Olasoji SO (2014) Assessment of heavy metal contamination of Dzindi River, in Limpopo Province, South Africa. Int J Nat Sci Res 2(10):185–194. https://archive.conscientiabeam.com/index.php/63/article/view/2330/3483
Embrapa (2006) Sistema brasileiro de classificação de solos. (2nd edn), Rio de Janeiro
Essington ME (2015) Soil and water chemistry: an integrative approach, 2nd edn. CRC Press, Boca Raton
Fan M, Shibata H (2015) Simulation of watershed hydrology and stream water quality under land use and climate change scenarios in Teshio River watershed, northern Japan. Ecol Ind 50:79–89. https://doi.org/10.1016/j.ecolind.2014.11.003
FAO (Food and Agriculture Organisation of the United Nations) (2007) Unlocking the water potential of agriculture. Natural Resources Management and Environment Department, Food and Agriculture Organization of the United Nations. URL:http://www.fao.org/docrep/006/y4525e/y4525e05.htm (Accessed on 12 February 2023)
Fatoki OS, Akinsoji OS, Ximba BJ et al (2013) Arsenic contamination: Africa the missing gap. Asian J Chem 25:9263–9268. https://doi.org/10.14233/ajchem.2013.15360
Fernandez-Luqueno F, Lopez-Valdez F, Gamero-Melo P, Luna-Suarez S, Aguilera-Gonzalez EN, Martínez AI et al (2013) Heavy metal pollution in drinking water-a global risk for human health: a review. Afr J Environ Sci Technol 7:567–584
Gardner JL, Al-Hamdani SH (1997) Interactive effects of aluminum and humic substances on Salvinia. J Aquat Plant Manag 35:30–34
Gevorgyan G, Mamyan A, Boshyan T, Vardanyan T, Vaseashta A (2021) Heavy metal contamination in an industrially affected river catchment Basin: assessment, effects, and mitigation. IJERPH 18:2881. https://doi.org/10.3390/ijerph18062881
Gqomfa B, Maphanga T, Phungela TT et al (2023) El Niño southern oscillation (ENSO) implication towards crocodile river water quality in South Africa. Sustainability 15:11125. https://doi.org/10.3390/su151411125
Hama Aziz KH, Mustafa FS, Omer KM, Hama S, Hamarawf RF, Rahman KO (2023) Heavy metal pollution in the aquatic environment: efficient and low-cost removal approaches to eliminate their toxicity: a review. RSC Adv 13:17595–17610. https://doi.org/10.1039/D3RA00723E
Hughes JP, Polissar L, Van Belle G (1988) Evaluation and synthesis of health effects studies of communities surrounding arsenic producing industries. Int J Epidemiol 17:407–413. https://doi.org/10.1093/ije/17.2.407
Inayat I, Batool AI, Rehman MFU, Ahmad KR, Kanwal MA, Ali R, Khalid R, Habib SS (2023) Seasonal variation and association of heavy metals in the vital organs of edible fishes from the River Jhelum in Punjab, Pakistan. Biol Trace Elem Res. https://doi.org/10.1007/s12011-023-03730-z
Jackson V, Paulse A, Odendaal J, Khan W (2009) Investigation into the metal contamination of the Plankenburg and Diep Rivers, Western Cape, South Africa. WSA 35. https://doi.org/10.4314/wsa.v35i3.76766
Jaishankar M, Tseten T, Anbalagan N et al (2014) Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol 7:60–72. https://doi.org/10.2478/intox-2014-0009
Kabata-Pendias A (2010) Trace elements in soils and plants. CRC Press, Boca Raton
Keshav Krishna A, Rama Mohan K, Murthy NN (2011) A multivariate statistical approach for monitoring of heavy metals in sediments: a case study from Wailpalli Watershed, Nalgonda District, Andhra Pradesh. India Res J Environ Earth Sci 3(2):103–113
Khallaf EA, MNAuthman M, AAlne-na-ei A (2021) Aluminum, chromium, and manganese in sediments of bahr shebeen nilotic Canal, Egypt: spatial and temporal distribution, pollution indices and risk assessment. Egypt J Aquat Biol Fish 25:983–1015. https://doi.org/10.21608/ejabf.2021.154320
Khan MZH, Hasan MR, Khan M et al (2017) Distribution of heavy metals in surface sediments of the Bay of Bengal Coast. J Toxicol 2017:1–7. https://doi.org/10.1155/2017/9235764
Korium MA, Toufeek MEF, El-Haty EY (2006) Distribution of Al, Ag, Cr, Mn, Ni, Zn and some physicochemical characteristics of River Nile water and sediment at Aswan. Egyp J Aquat Res 32(2):208–225
Kusimi JM, Kusimi BA (2012) The hydrochemistry of water resources in selected mining communities in Tarkwa. J Geochem Explor 112:252–261. https://doi.org/10.1016/j.gexplo.2011.09.003
Li X, Lee S, Wong S et al (2004) The study of metal contamination in urban soils of Hong Kong using a GIS-based approach. Environ Pollut 129:113–124. https://doi.org/10.1016/j.envpol.2003.09.030
Lipy EP, Hakim M, Mohanta LC, Islam D, Lyzu C, Roy DC, Jahan I, Akhter S, Raknuzzaman M, Abu Sayed Md (2021) Assessment of Heavy metal concentration in water, sediment and common fish species of Dhaleshwari River in Bangladesh and their health implications. Biol Trace Elem Res 199:4295–4307. https://doi.org/10.1007/s12011-020-02552-7
lta Erebho U (2022) Relationship between electrical conductivity and some mineral composition of Benin Rivers. Pak J Sci Ind Res A Phys Sci 65(2):104–111
Marín-Spiotta E, Gruley KE, Crawford J et al (2014) Paradigm shifts in soil organic matter research affect interpretations of aquatic carbon cycling: transcending disciplinary and ecosystem boundaries. Biogeochemistry 117:279–297. https://doi.org/10.1007/s10533-013-9949-7
Mitra S, Chakraborty AJ, Tareq AM, Emran TB, Nainu F, Khusro A, Idris AM, Khandaker MU, Osman H, Alhumaydhi FA, Simal-Gandara J (2022) Impact of heavy metals on the environment and human health: novel therapeutic insights to counter the toxicity. J King Saud Univ Sci 34:101865. https://doi.org/10.1016/j.jksus.2022.101865
Moyosore JO, Sridhar MKC, Coker AO, Mumuni A (2014) Iron and manganese levels of groundwater in selected areas in Ibadan and feasible engineering solutions. Eur Sci J 10:137–153
Muhammad S (2023) Evaluation of heavy metals in water and sediments, pollution, and risk indices of Naltar Lakes, Pakistan. Environ Sci Pollut Res 30:28217–28226. https://doi.org/10.1007/s11356-022-24160-9
Pagano T, Bida M, Kenny J (2014) Trends in levels of allochthonous dissolved organic carbon in natural water: a review of potential mechanisms under a changing climate. Water 6:2862–2897. https://doi.org/10.3390/w6102862
Pal D, Maiti SK (2018) Seasonal variation of heavy metals in water, sediment, and highly consumed cultured fish (Labeo rohita and Labeo bata) and potential health risk assessment in aquaculture pond of the coal city, Dhanbad (India). Environ Sci Pollut Res 25:12464–12480. https://doi.org/10.1007/s11356-018-1424-5
Raghunath R, Tripathi RM, Kumar AV et al (1999) Assessment of Pb, Cd, Cu, and Zn exposures of 6- to 10-year-old children in Mumbai. Environ Res 80:215–221. https://doi.org/10.1006/enrs.1998.3919
Rango T, Vengosh A, Dwyer G, Bianchini G (2013) Mobilization of arsenic and other naturally occurring contaminants in groundwater of the main Ethiopian Rift aquifers. Water Res 47:5801–5818. https://doi.org/10.1016/j.watres.2013.07.002
Rani L, Srivastav AL, Kaushal J, Grewal AS, Madhav S (2022) Heavy metal contamination in the river ecosystem. Ecol Signif River Ecosyst. https://doi.org/10.1016/B978-0-323-85045-2.00016-9
Reza R, Singh G (2010) Heavy metal contamination and its indexing approach for river water. Int J Environ Sci Technol 7:785–792. https://doi.org/10.1007/BF03326187
Rim-Rukeh A, Ikhifa OG, Okokoyo AP (2006) Effects of agricultural activities on the water quality of Orogodo river, Agbor Nigeria. J Appl Sci Res 2:256–259
Rostami S, He J, Hassan Q (2018) Riverine water quality response to precipitation and its change. Environments 5:8. https://doi.org/10.3390/environments5010008
Saleem M, Iqbal J, Shah MH (2019) Seasonal variations, risk assessment and multivariate analysis of trace metals in the freshwater reservoirs of Pakistan. Chemosphere 216:715–724. https://doi.org/10.1016/j.chemosphere.2018.10.173
Sander M, Hofstetter TB, Gorski CA (2015) Electrochemical analyses of redox-active iron minerals: a review of nonmediated and mediated approaches. Environ Sci Technol 49:5862–5878. https://doi.org/10.1021/acs.est.5b00006
Schiedek D, Sundelin B, Readman JW, Macdonald RW (2007) Interactions between climate change and contaminants. Mar Pollut Bull 54:1845–1856. https://doi.org/10.1016/j.marpolbul.2007.09.020
Senze M, Kowalska-Góralska M, Czyż K (2021a) Availability of aluminum in river water supplying dam reservoirs in Lower Silesia considering the hydrochemical conditions. Environ Nanotechnol Monit Manag 16:100535. https://doi.org/10.1016/j.enmm.2021.100535
Senze M, Kowalska-Góralska M, Czyż K et al (2021b) Aluminum in bottom sediments of the Lower Silesian Rivers supplying dam reservoirs vs selected chemical parameters. IJERPH 18:13170. https://doi.org/10.3390/ijerph182413170
Serfor-Armah Y, Nyarko BJB, Dampare SB, Adomako D (2006) Levels of arsenic and antimony in water and sediment from Prestea, a gold mining town in Ghana and its environs. Water Air Soil Pollut 175:181–192. https://doi.org/10.1007/s11270-006-9127-9
Shafi J, Mirza ZS, Kosour N, Zafarullah M (2018) Assessment of water quality and heavy metals contamination of River Ravi in Pakistan. Pak J Anal Environ Chem 19:169–180. https://doi.org/10.21743/pjaec/2018.12.19
Shafiq HB, Ajaz M, Rasool SA (2011) Bacterial and toxic pollutants in lakes of river. Indus Pak J Bot 43:1765–1772
Siddiquee S, Rovina K, Azad SA (2015) Heavy metal contaminants removal from wastewater using the potential filamentous fungi biomass: a review. J Microb Biochem Technol 7:384–395. https://doi.org/10.4172/1948-5948.1000243
Singh N, Kumar D, Sahu A (2007) Arsenic in the environment: effects on human health and possible prevention. J Environ Biol 28:359–365
Siraj M, Khisroon M, Khan A (2016) Bioaccumulation of heavy metals in different organs of Wallago attu from River Kabul Khyber Pakhtunkhwa, Pakistan. Biol Trace Elem Res 172:242–250. https://doi.org/10.1007/s12011-015-0572-4
Sunda WG (2012) Feedback interactions between trace metal nutrients and phytoplankton in the ocean. Front Microbiol 3:204. https://doi.org/10.3389/fmicb.2012.00204
Tet Nay Tun M (2003) Arsenic contamination of water sources in rural Myanmar. Towards the millennium development goals. In: 29th WEDC international conference. Abuja, Nigeria
Turpeinen R, Kairesalo T, Häggblom MM (2004) Microbial community structure and activity in arsenic-, chromium-and copper-contaminated soils. FEMS Microbiol Ecol 47:39–50. https://doi.org/10.1016/S0168-6496(03)00232-0
Wei H, Wang Y, Liu J, Zeng R (2022) Heavy metal in river sediments of Huanghua city in water diversion area from Yellow River, China: contamination, ecological risks, and sources. Water 15:58. https://doi.org/10.3390/w15010058
Wong CSC, Li XD, Zhang G et al (2003) Atmospheric deposition of heavy metals in the Pearl River Delta, China. Atmos Environ 37:767–776. https://doi.org/10.1016/S1352-2310(02)00929-9
World Health Organization (2021) A global overview of national regulations and standards for drinking-water quality
Wright D, Welbourne (2002) Environmental toxicology. Factors affecting toxicology. Cambridge Environmental Chemistry Series 11. Cambridge University Press, Cambridge
Wu YF, Liu CQ, Tu CL (2008) Atmospheric deposition of metals in TSP of Guiyang, PR China. Bull Environ Contam Toxicol 80:465–468. https://doi.org/10.1007/s00128-008-9397-6
Xia XH, Wu Q, Mou XL, Lai YJ (2015) Potential impacts of climate change on the water quality of different water bodies. J Env Inform 25:85–98. https://doi.org/10.3808/jei.201400263
Ying SC, Schaefer MV, Cock-Esteb A et al (2017) Depth stratification leads to distinct zones of manganese and arsenic contaminated groundwater. Environ Sci Technol 51:8926–8932. https://doi.org/10.1021/acs.est.7b01121
Zhai Y, Cao X, Xia X et al (2021) Elevated Fe and Mn concentrations in groundwater in the Songnen Plain, Northeast China, and the factors and mechanisms involved. Agronomy 11:2392. https://doi.org/10.3390/agronomy11122392
Funding
Open access funding provided by Cape Peninsula University of Technology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
We are solely responsible for the manuscript’s veracity, validity, and originality, and we are the sole owners of its contents. Additionally, we certify that this manuscript is our original work and has not been plagiarized. My manuscript is completely original.
Consent for publication
We certify that the manuscript you have received has not been published elsewhere or is under consideration by any other journals.
Additional information
Editorial responsibility: Samareh Mirkia.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Maphanga, T., Chidi, B.S., Phungela, T.T. et al. The interplay between temporal and seasonal distribution of heavy metals and physiochemical properties in Kaap River. Int. J. Environ. Sci. Technol. 21, 6053–6064 (2024). https://doi.org/10.1007/s13762-023-05401-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-023-05401-x