Abstract
This research investigated the feasibility of employing organic acids, like citric acid, produced by Aspergillus nidulans MT355567 in a bioleaching process to recover uranium (U) from a low-grade rock sample. The optimal conditions for fungal growth and maximum citric acid (CA) synthesis across three distinct media were determined. The maximum citric acid concentration was produced on medium made from wheat bran (83%) and tea waste (77%). An investigation was carried out to see how citric acid and, by consequence, uranium bioleaching affinity, were affected by varying carbon sources, nitrogen sources, pH, temperature, incubation period, ore particle size, and the solid–liquid ratio. At 25 °C and a pH of 5.0, media containing 100 g/L of sucrose as a carbon source and peptone as a nitrogen source made the highest yield of citric acid and U bioleaching. Higher U bioleaching was achieved with ore particles 0.075 mm at a ratio of 2 g/L after only 30 min of contact with the fungal filtrate. Iron interference has a negative impact on uranium extraction. Interestingly, none of the conditions applied to enhance CA synthesis and U-bioleaching caused iron (Fe) dissolution. Based on these findings, it appears that bioleaching using A. nidulans MT355567 metabolic products is a promising economic and ecofriendly technology for extracting uranium from low-grade ore that might be adopted on a pilot scale.
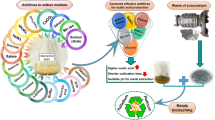
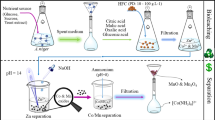
Graphical abstract
summarizing the experimental workflow for bioleaching of uranium from low-grade ore using citric acid produced by Aspergillus nidulans. The process involved optimizing A. nidulans growth and citric acid biosynthesis, evaluating factors influencing bioleaching activity of the acid metabolite solutions, and finally applying the optimized conditions to bioleach uranium from the ore sample. The schematic illustrates the key steps and the optimal condition for Aspergillus nidulans growth medium preparation using agricultural wastes, downstream application of metabolite synthesized for uranium bioleaching

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Historically, uranium has served as a source of atomic energy in the defense and power industries, as well as a source of alternative energy in the maritime industry. Growing uranium consumption has resulted to the eventual depletion of tall review and easily leachable ore deposits (Abostate et al. 2018; Sun et al. 2020). A progressive depletion of high-grade uranium reserves has resulted from the increased demand for nuclear energy. New technology for extracting low-grade and refractory uranium ore must be developed in order to meet the rising demand for this scarce material (Zhou et al. 2019). Uranium is a naturally occurring element with an average concentration of 2.8 parts per million in the Earth's crust. Traces of it occur almost everywhere.
Uranium is a trace naturally occurring element with an average concentration in the Earth's crust of 2.8 parts per million. The Nuclear Energy Agency in Paris estimates that Egypt has less than 0.01% of the world's identifiable uranium reserves, which is insufficient to generate commercial quantities that can be exported profitably. Most uranium deposits in Egypt contain low-grade uranium ores that are amenable to leach (Abdel-Monem et al. 1996; Nuclear Energy Agency (NEA) 2014).
Most of the uranium in rock was consumed by conventional leaching methods; therefore, it is necessary to add additional resources or re-extract uranium from waste to safeguard the environment from its toxicity and use it in the energy sector.
To offset the negative effects of metal resource extraction, the global community is promoting environmentally friendly mining technology (Krishnamoorthy et al. 2021; Dusengemungu et al. 2021). Bioleaching is being investigated as a viable alternative to hydro- and pyrometallurgical metal recovery techniques since it is a cost-effective process. The process can utilize shake flasks, batch reactors, continuous reactors, columns, and heaps. Bioleaching has been identified as a method for recovering these resilient uranium metals in a productive, temperate, and ecologically friendly manner (Rasoulnia et al. 2021). Typically, heterotrophic bacteria, autotrophic bacteria, and fungal species are used for bioleaching. Aspergillus species are the most efficient bioleaching fungi. These fungi create organic acids, and the dissociation of organic acids leads to the elimination of protons. Compared to H2SO4 and other strong acids, the organic acids produced by microbes are largely non-corrosive and therefore ecofriendly. Iron and sulpur oxidizing bacteria are frequently used in the bioleaching of sulpide ores because they use the ore's iron and sulpur as a substrate and carbon dioxide as a carbon source for their growth. Metal dissolution can occur in a variety of ways, depending on the type of microorganism and the type of mineral (Krishnamoorthy et al. 2021).
Under leaching conditions, both soluble and insoluble organic-uranium complexes exist, such as citric-uranium complexes and oxalic-uranium complexes, respectively. Oxalic acid has a greater degree of hydrogen liberation than other organic acids and a stronger chemical affinity in the aqueous phase, both of which facilitate uranium leaching (Wang et al. 2015). Extraction of metals from nonsulfidic minerals can be catalyzed by organisms, yeasts, or heterotrophic microorganisms that can frequently tolerate high pH values. These microorganisms produce vast quantities of natural acids, including citric, lactic, gluconic, and tartaric acids, among others; these metabolites can liberate and mobilize metals from nonsulfidic solids, resulting in metal dissolution. Aspergillus niger's production of oxalic acid is dependent on the pH of the medium and the carbon source. Since the activity of oxaloacetate acetylhydrolase (OAH), the protein thought to be responsible for oxalate arrangement, is at its peak at pH 6, its production can proceed, whereas below pH 2 no oxalic acid is produced (Sawant et al. 2018). Acid-producing fungi, including Aspergillus, Penicillium, Phanerochaete, and Chrysosporium, have leached metals such as copper, nickel, aluminum, uranium, etc. (Das et al. 2019; Dusengemungu et al. 2021; El-Bondkly and El-Gendy 2022).
Bioleaching of metals with microorganisms can be ‘‘one-step’’ when microorganism growth and metal dissolution occur together. Otherwise, ‘‘two-step’’, the microorganism is allowed to grow then the metal is included to medium containing metabolites generated already by the microorganism. The "two-step" bioleaching method is the most effective for a number of reasons: first, the production of the microorganism's metabolites is increased when it is grown in optimal conditions without the presence of metal. Second, the optimal conditions for microorganism growth and metal leaching are distinct. The handling of each of these steps can subsequently be optimized. Besides, microorganism development is usually initiated in neutral pH and utilizes natural substrate in sterile environment, sterilizing of mineral in industry is not conceivable. Some species such as heterotrophic microscopic organisms may impact contagious development and change the anticipated way, they work (Gerayeli et al. 2013).
Citric acid (CA), a natural Kreb's cycle metabolic intermediate, is commonly regarded as safe, nontoxic, and biodegradable in addition to its involvement in bioleaching. Due to these features, it has extensive application in numerous fields, including the food, dairy, pharmaceutical, cosmetic, agricultural, and biochemical industries. Its antioxidant properties make it useful in a wide range of healthcare products. There is an increasing demand for CA in the biomedical industry, where it is used as an active ingredient in the creation of a wide variety of biopolymers with applications ranging from nanomedicines and drug delivery systems to the cultivation of a wide variety of human cell lines and the solubilization of metals (Max et al. 2010; Behera et al. 2021).
The goal of this study is to develop a low-cost alternative growth medium for A. nidulans using agricultural residues (tea waste and wheat bran) to be employed in U bioleaching. The presence of ferrous iron (Fe +2) and metallic iron (Fe0) reduces U(VI) to the insoluble form U(IV), with uranium adsorbed on the iron surface (Dublet et al. 2017). Therefore, the dissolution of U without Fe is a valuable target. Here, we evaluated the ability to bioleach U without simultaneously dissolving Fe, which increases the purity of the bioleached U. An optimization study investigated the most influential variables on U bioleaching and CA production, including carbon source, nitrogen source, pH, temperature, incubation period, ore particle size, and solid–liquid ratio.
Materials and methods
Materials
The uranium ore sample
The ore material containing the uranium element that used in the current study was collected from Abu Zarab in Sinai, Egypt. The composition of uranium and other element oxides in the used ore sample was detected as mentioned below in the methods section and tabulated in Table 1.
Microorganism and growth media
A. nidulans was isolated from monazite mineral collected from Egypt's Rosetta region on the Mediterranean coast. By sequencing the ITS region of the rRNA-encoding gene, the fungal isolate was identified. The sequence was submitted to the NCBI gene bank with the accession number “MT355567” (Abozaid et al. 2021).
Potato dextrose agar plates were used for the routine maintenance of the fungal culture. Sabouraud dextrose (Guinea et al. 2005), wheat bran (WB) and tea waste (TW) were used to determine the optimal culture medium for better uranium bioleaching. Wheat bran flour (70% extraction) was obtained from the local market and used directly without refining.
Chemicals
All chemicals used for bioleaching experiments were analytical-grade, Merck, life science, industrial and lab chemicals products (https://www.merckmillipore.com/INTL/en). The aqueous solutions were prepared with distilled water.
Instrumentation
The concentration of uranium and other major oxides was measured in ore sample using Spectrometer T80 UV/VIS Spectrometer (PG Instruments Ltd). All pH measurements were measured by using a calibrated pH meter (Jenway 3510) equipped with a Standard Calomel Electrode (SCE). Electromagnetic Sieve Shaker- HAVER EML 200 digital T- Haver and Boecker was used to obtain different grain sizes (ranged from 0.25 to 0.025 mm).
Biochemical and chemical methods
Determination of citric acid production and protein contents
Citric acid (CA) is one of the most common components of fungal metabolites and involved in uranium leaching from ore sample (Xu et al. 2019). Therefore, the citric acid concentration was evaluated as an indicator for uranium leaching efficiency. It was determined titrimetrically in the filtrate by using 0.1N NaOH and phenolphthalein as indicator and calculated as % according to the following formula (AOAC 2002).
\(\mathrm{\% CA}\hspace{0.17em}=\hspace{0.17em}\frac{\mathrm{Normality\; of\; NaOH }\;\times\; \mathrm{ volume \;of \;NaOH }\;\times \;\mathrm{ Equiv}.\mathrm{ wt}.\;\mathrm{ of\; CA}}{\mathrm{Weight \;of \;sample}\;\times\; 100}\)
The fungal extracellular proteins were determined according to (Gregor et al. 1977; Dawnay StJ. et al. 1991).
Determination of uranium and major oxides concentrations in the ore sample
The uranium (U) concentration in the leach liquor was quantified using volumetric titration with ammonium vanadate, following the method of Davies and Gray (1964). The concentrations of the major oxide compounds in the leach liquor were determined using wet chemical procedures according to the method described by Shapiro and Brannock (1962).
The bioleaching experiment
Bioleaching experiments were performed using a batch system. The experiments were carried out using seven days old A. nidulans biomass-free filtrate mixed with sterilized uranium-containing ore sample in ratio (1 g/L). The mixture was mixed for maximum 3 h contact time at room temperature in an orbital shaker at 150 rpm. Wheat bran (2 g/L) or tea waste (2 g/L) media was used for the bioleaching optimization experiments as they afford the best fungal growth and uranium bioleaching.
Bioleaching optimization factors
The uranium bioleaching process was optimized using various factors such as various carbon sources and doses, various nitrogen sources, different buffer types, various time periods, different temperatures, different grain sizes and various solid liquid ratios. The experiment components were shaken at 150 rpm and the concentration of uranium was measured every time to select the optimized conditions for uranium leaching (see Graphical abstract).
Effect of carbon source and dose
Glucose, sucrose, and starch were used as different carbon sources. Experiments were carried out in 250 ml sterilized conical flasks containing 50 ml of 2 g/L wheat bran or tea waste media in addition to (50 g/L) of the corresponding carbon source. The effect of different sucrose concentrations (50, 100, and 150 g/L) was also investigated under the above-mentioned experimental condition, as it was the best carbon source.
Effect of different nitrogen sources
Different nitrogen sources such as Peptone, yeast extract, beef extract and urea were evaluated for the maximum uranium bioleaching. Ten grams of each corresponding source was provided separately to one liter of the media.
Effect of different buffer types
Different buffers were employed to alter the medium pH, and the buffer solutions (acetate (pH 5.6), phosphate (pH 5.6) and Macllivian (pH 5) were used.
Effect of different temperatures
Seven days old A. nidulans biomass-free filtrate was mixed with sterilized uranium-containing ore sample in ratio (1 g/L). The mixture was mixed for 3 h contact time at different temperature 15, 20, 25, 30 and 35 °C) in an orbital shaker at 150 rpm.
Effect of different contact time
The effect of fungal filtrate contact time with the ore sample on the U bioleaching efficiency was also assessed at different time periods (15, 30, 45, 90, 120, and 180 min).
Effect of different grain (ore particles) sizes
The ore sample was grinded to a fine powder using mortar and pestle. The powder was sieved using a sieve set with pore size ranging from (0.25 to 0.025) mm. The collected particles from each sieve were used separately in each experiment.
Effect of different solid/liquid ratios
Different ratios of ore particles (solid) to fungal filtrate (liquid) were used to assess the influence on U bioleaching. The following ratios (1: 1, 2:1, 3:1, 4:1, and 5:1 g/L) were used at the best measured conditions.
Results and discussion
Cost-effective substrates for A. nidulans CA production and U bioleaching
In the current study, wheat bran or tea waste were used as a substrate for A. nidulans growth in comparison to the routine used media (Sabouraud dextrose medium). Both natural substrates provide better growth as indicated by the fungal protein content which reaches up to maximum of 3.3 and 3.1 g/l respectively. Also, both substrates provide a superior CA production and U bioleaching affinity, although, using media containing wheat bran provides the highest values. Therefore, wheat bran and tea waste containing media were used for the whole study (Fig. 1).
Wheat grains and wheat bran were the best substrates for the growth of Morchella spongiola (MR17) with high lignocellulolytic activity. Wheat grains yielded a protein content = 17.4 mg/ml, while wheat bran yielded only 11.2 mg/ml. (Reddy and Kanwal 2022).
Chemical composition of the used ore sample
A. nidulans affinity to bioleach U from low grade ore sample was assessed, the chemical analysis of ore sample has showed that the SiO2 was the predominant metal oxide of 49.1% with trace amounts of 0.3% MgO was present of the total composition. Al2O3 and Fe2O3 are, respectively, the second and third most abundant metal oxides in the ore sample (Table 1).
Wang et al. (2015) bioleached uranium from a sample of uranium ore from a uranium deposit in West China. The chemical composition was like our ore sample, but with different proportions: 85.44 percent of their ore sample was SiO2, followed by 6.56 percent Al2O3 and 1.49 percent Fe2O3. U constitutes 0.04% of our sample, but 0.118% of the ore sample from west China; hence, the ore is a readily leachable uranium ore.
Effect of carbon sources on uranium bioleaching
In the current study, 50 g/L of glucose, sucrose or starch were used to select the best carbon source for uranium bioleaching. The results showed that the sucrose was the best for uranium bioleaching reached 67.32 and 66.83% in comparison to 30.94 and 37.12% for starch, in both substrates: wheat bran and tea waste medium respectively (Fig. 2).
Effect of different carbon sources on citric acid (CA) production and uranium (U) bioleaching activity by Aspergillus nidulans. The fungus was cultured in wheat bran (WB) and tea waste (TW) media supplemented with different carbon sources: glucose, sucrose, or starch. Citric acid concentrations in the culture filtrates were quantified. The bioleaching activity was evaluated by determining the percent of uranium extracted after treating the low-grade uranium ore sample for 180 min contact time at 25 °C
The pH of the media was decreased from 5.6 to 4.5 and 4 for wheat bran and tea waste media respectively, indicating citric acid production. Citric acid (CA) is one of the most common components of fungal metabolites and has a role in uranium leaching from ore sample (Xu et al. 2019). With measuring citric acid levels, we found an increase of up to 38 and 33% for fungi cultured on wheat bran and tea waste, respectively, accompanied by an increase in protein concentration of 1.1 and 1 mg/ml. The type of carbon source has a substantial effect on the growth and citric acid generation capability of fungi (Das et al. 2019). Due to the existence of extracellular-bound invertase that is active at low pH and rapidly hydrates sucrose into glucose and fructose, sucrose is favored over glucose and fructose (Sati and Bisht 2006; Shah et al. 2020) As the conversion of polysaccharides into simple sugar involves additional steps, they are not favored as a carbon source. It takes a substantial amount of time for the decomposition process to attain the sugar catabolism rate required for citric acid production. The increased protein content of the fungus cultured on a medium containing sucrose may represent the fungus' ability to create extra enzymes necessary for citric acid synthesis. The acidity of the medium facilitates metal leaching from any substance. Reduced enzymatic activity influences the pH of the fermentation medium, which slows the rate of polysaccharide breakdown (Das et al. 2019).
Increasing the sucrose concentration to 100 g/L increased the fungus protein content in wheat bran and tea waste containing medium to 1.5 and 1.2 mg/ml, citric acid production to 45 and 42%, and bioleaching ability to 71.78 and 69.31%, respectively. A slight difference was observed between 100 and 150 g/L of sucrose (Fig. 3). Previously, a correlation between sugar concentration and citric acid production by Aspergillus niger was identified. Two date varieties with varying sugar contents were added to a medium inoculated with A. niger for the production of citric acid. The GHARS variety with a higher sugar content produced 0.32 g/g citric acid, while the MECH DEGLA variety with a lower sugar content produced 0.28 g/g citric acid (Chergui et al. 2021). In a different study, date syrup with a higher sugar concentration produced an even greater amount of CA, 80.6 g/L (Mostafa and Alamri 2012).
Effect of different sucrose concentrations on citric acid (CA) production and uranium (U) bioleaching activity by Aspergillus nidulans. The fungus was cultured in wheat bran (WB) and tea waste (TW) media supplemented with different concentrations of sucrose (50, 100 or 150 g/L). Citric acid concentrations in the culture filtrates were quantified. The bioleaching activity was evaluated by determining the percent of uranium extracted after treating the low-grade uranium ore sample for 180 min contact time at 25 °C
Effect of nitrogen sources
Different nitrogen sources such as Peptone, yeast extract, beef extract and urea were evaluated for the improved uranium bioleaching. The best nitrogen source for wheat bran medium was peptone with CA production level of 40%, and the uranium leaching was 64.35%. While yeast extract was the best for tea waste medium with 33% citric acid production level and 63.11% uranium leaching efficiency, (Fig. 4).
Effect of different nitrogen sources on citric acid (CA) production and uranium (U) bioleaching activity by Aspergillus nidulans. The fungus was cultured in wheat bran (WB) and tea waste (TW) media supplemented with 100g/L sucrose as a carbon source and different nitrogen sources: peptone, yeast extract, urea, or beef extract. Citric acid concentrations in the culture filtrates were quantified. The bioleaching activity was evaluated by determining the percent of uranium extracted after treating the low-grade uranium ore sample for 180 min contact time at 25 °C
Nitrogen has a significant impact on citric acid production, as it is not only essential for cellular metabolism but also a fundamental component of cell proteins. In CA production, ammonium salts such as ammonium nitrate and sulpate, urea, peptone, malt extract, etc. are utilized (Behera et al. 2021). Because the consumption of acid ammonium compounds results in a decrease in pH, which is essential for citric fermentation, these compounds are preferred. In the oleaginous yeast Yarrowia lipolytica, growth on a complex nitrogen source such as peptone enables rapid growth with limited accumulation of lipids droplets, whereas growth on a simple nitrogen source such as ammonium promotes accumulation of lipids in large lipid droplets (Pomraning et al. 2017). Aspergillus niger isolated from the leaf litter soil of Sathuragiri Hills produced different amounts of citric acid using various nitrogen sources. Various concentrations of citric acid were produced (10.22 ± 0.05, 9.13 ± 0.07, 6.36 ± 0.04 g/L) for ammonium chloride, ammonium sulpate and urea respectively which were used as nitrogen sources (Shankar and Sivakumar 2016). Depending on the nitrogen source, the production of citric and gluconic acid by Aspergillus niger, Penicillium bilaii, P. simplicissimum, and Paxillus involutus resulted in different solubilization activities for natural gypsum (Gharieb and Gadd 1999). Enzymes play a crucial role in the formation of CA. Inhibition of some enzymes during glycolysis causes high flux of citric acid that results in its accumulation by this study. Nitrogen deficiency inhibits the anabolism of the fungus, resulting in protein degradation and an increase in intracellular NH4+ concentration. This high intracellular NH4+ concentration inhibits an essential enzyme in the glycolysis pathway, resulting in the accumulation of carbonic anhydrase (CA), which increases the bioleaching activity of the fungus (Behera et al. 2021).
Effect of different buffer types
The pH level has a significant effect on fungal growth and citric acid production. A direct correlation was observed between the ability of Aspergillus niger to produce citric acid and the pH value of the medium (Shankar and Sivakumar 2016).
The best buffer for the fungus cultured in wheat bran was Mcllivain which accompanied with high citric acid production of 63% and uranium leaching of 74%, while the best buffer for tea waste was phosphate buffer which accompanied with high citric acid production of 60% and uranium leaching of 71.78%. As shown in Fig. 5. The current outcome conforms to Shankar and Sivakumar (2016). During the sporulation and production phases, the pH of the fermentation medium is crucial. Microbes such as Aspergillus, Rhizopus, and Penicillium species can rapidly lower the pH to below 3, whereas Sporotrichum and Pleurotus species produce a more stable pH between 4 and 5 (Del Campo et al. 2006) Developing spores absorb ammonia and release protons during the germination phase, increasing the acidity of the medium and promoting citric acid synthesis. At a pH of less than 2, the formation of undesirable products such as oxalic and gluconic acid is inhibited, and the risk of contamination by other microorganisms is reduced, making citric acid recovery easier (Sawant et al. 2018). Protein production was 3 mg/ml for the fungus cultured on wheat bran and 2.4 mg/ml for tea waste cultured fungus after growth in Mcllivain buffer.
Effect of different buffer types on citric acid (CA) production and uranium (U) bioleaching activity by Aspergillus nidulans. The fungus was cultured in wheat bran (WB) and tea waste (TW) media supplemented with 100 g/L sucrose as a carbon source, peptone for WB, yeast extract for TW as a nitrogen source. Citric acid concentrations in the culture filtrates were quantified. The bioleaching activity was evaluated by determining the percent of uranium extracted after treating the low-grade uranium ore sample for 180 min contact time at 25 °C
It was observed that the concentration of citric acid increased with an acidic pH of 5.5 and then gradually decreased with the growth of fungi. At a pH of 5.5, the optimal citric acid concentration of 1.94 g/L was obtained from corn starch hydrolysate using Aspergillus Niger (Amenaghawon and Aisien 2012). The pH is significant in two ways. First, for spore germination, this is necessary for fermentation, a pH of 5 or higher is required. Second, when ammonia is absorbed by germinating spores, protons are released. This results in the release of hydrogen ions, which lowers the medium's pH. The low pH has the effect of increasing citric acid production and creating an environment that is close to sterile, thereby decreasing the risk of contamination. At pH5, the production of citric acid was 66.7% (Narayanamurthy et al. 2008; Shankar and Sivakumar 2016).
Effect of different temperature
The highest citric acid production by Aspergillus nidulans was observed at 25 °C, with 83 and 77% citric acid production for wheat bran and tea waste-based culture mediums, respectively, and 81.6 and 74.25% uranium leached for wheat bran and tea waste. In contrast, citric acid production and uranium leaching were lowest at 35 °C (Fig. 6).
Effect of different temperatures on citric acid (CA) production and uranium (U) bioleaching activity by Aspergillus nidulans. The fungus was cultured in wheat bran (WB) and tea waste (TW) media supplemented with 100 g/L sucrose as a carbon source, peptone for WB, yeast extract for TW as a nitrogen source, Mcllivain buffer for WB, phosphate buffer for TW, at different temperatures. Citric acid concentrations in the culture filtrates were quantified.The bioleaching activity was evaluated by determining the percent of uranium extracted after treating the low-grade uranium ore sample for 180 min contact time at (15–25 °C)
A similar effect of temperature was observed in a study, with citric acid production increasing between 25 and 30 °C, followed by a decline in production. The fermentation temperature is significant because cells cultured at temperatures below ideal exhibit signs of poor growth and metabolic production (Dunya A Alhadithy 2020). When Aspergillus niger cells are incubated at low or high temperatures, citric acid synthesis is affected by slow fungal germination, slow metabolic activity, enzyme denaturation, and decreased cell viability. The ideal temperature found in this study is consistent with the fact that filamentous fungus, such as A. niger, are mesophilic, needing temperatures between 25 and 35 °C for optimal growth (Rinu and Pandey 2010).
The temperature of the fermentation medium is one of the most important parameters affecting citric acid synthesis. Upon growing the fungus at 25 °C, pH of both waste media decreased to 3 for wheat bran and 3.5 for tea waste medium. While, protein content was 3.2 and 3 mg/ml for the fungus cultured on wheat bran and tea waste media respectively.
CA production is dependent on the enzymes of glycolysis and the TCA cycle. These enzymes are temperature dependent. When the temperature of the medium was low, so was the enzyme activity, which had no effect on the production of citric acid. However, when the temperature of the medium exceeded 30 °C, citric acid biosynthesis decreased. It may be due to the accumulation of byproducts such as oxalic acid, while temperatures below 25 °C reduced the growth of the organism and fermentation rates (Arzumanov et al. 2000).
Effect of different contact time
To determine the optimal time for uranium leaching, the contact time between the fungal filtrate and the ore sample was varied from fifteen to one hundred and eighty minutes. The optimal contact time was thirty minutes, which improved uranium leaching by 84.16% for wheat bran fungal filtrate and 79.22% for tea waste (Fig. 7). After 30 min of experimentation, uranium leaching stabilizes, possibly due to the consumption of citric acid and other bioactive compounds required for the bioleaching process. However, observation showed this rapid response, which is dependent on the metabolites produced. This is due to the fact that the fungal filtrate was used, which already contains the necessary metabolites for U bioleaching. Using a one-step leaching strategy, Xu et al. (2019) determined that citric acid production and U leaching taking a long time reached up to 20 days.
Effect of different contact time (min) between ore sample and the fungal filtrate on citric acid (CA) production and uranium (U) bioleaching activity by Aspergillus nidulans. The fungus was cultured in wheat bran (WB) and tea waste (TW) media supplemented with 100 g/L sucrose as a carbon source, peptone for WB, yeast extract for TW as a nitrogen source, Mcllivain buffer for WB, phosphate buffer for TW. Citric acid concentrations in the culture filtrates were quantified. The bioleaching activity was evaluated by determining the percent of uranium extracted after treating the low-grade uranium ore sample for (15–180 min) contact time at 25 °C
Effect of particle size
Using a series of particle sizes, the effect of ore particle size on uranium solubilization by fungal extract was studied (0.25–0.025 mm). In all mediums, WB and TW, a strong leaching ability for the fungal filtrate was identified for all particle sizes examined; however, WB performed somewhat better. The particle size with the maximum CA production and best U bioleaching ability was 0.075 mm, with bioleaching 91.58 and 84.15 percent for wheat bran and tea waste-based cultures, respectively (Fig. 8). The small particle size is desirable because it increases the surface area of the intact portion of the ore with the filtrate containing fungal metabolites, hence increasing the bioleaching capability. In the solution, particles less than 0.07 mm in diameter accumulated into a flat structure. This would lower the surface area between the metal and the fungal filtrate, hence decreasing the efficacy of bioleaching (Mezaguer et al. 2013; Abostate et al. 2018; Abozaid et al. 2021).
Effect of different particle size of the ore sample (mm) on citric acid (CA) production and uranium (U) bioleaching activity by Aspergillus nidulans. The fungus was cultured in wheat bran (WB) and tea waste (TW) media supplemented with 100 g/L sucrose as a carbon source, peptone for WB, yeast extract for TW as a nitrogen source, Mcllivain buffer for WB, phosphate buffer for TW. Citric acid concentrations in the culture filtrates were quantified. The bioleaching activity was evaluated by determining the percent of uranium extracted after treating the low-grade uranium ore sample (0.25–0.025 mm particles) for 30 min contact time at 25 °C
Effect of solid liquid ratio (S/L):
Using varying ranges (1:1–5:1 w/v), the influence of ore particle weight (S) on the fungal filtrate (L) employed in U bioleaching was examined. In both WB and TW, a substantial leaching ability was seen for the fungal filtrate at all investigated S/L ratios; however, WB performed slightly better. Wheat bran and tea waste-based cultures had bioleaching percentages of 94.05 and 89.11 percent, respectively, with an optimum S/L ratio of 2:1 g/L for maximum CA production and U bioleaching ability (Fig. 9). Higher S/L ratios demonstrate comparable CA generation and U bioleaching affinity. This could be due to the consumption of acid and other bioactive chemicals necessary for the bioleaching process with the requisite fungal culture, and any further ore particles would not find any acids in the reaction media to solubilize the extra U (Abostate et al. 2018; Abozaid et al. 2021).
Effect of different solid: liquid ratio of the ore sample (g) to the fungal filtrate (L) on citric acid (CA) production and uranium (U) bioleaching activity by Aspergillus nidulans. The fungus was cultured in wheat bran (WB) and tea waste (TW) media supplemented with 100 g/L sucrose as a carbon source, peptone for WB, yeast extract for TW as a nitrogen source, Mcllivain buffer for WB, phosphate buffer for TW. Citric acid concentrations in the culture filtrates were quantified. The bioleaching activity was evaluated by determining the percent of uranium extracted after treating the low-grade uranium ore sample (0.075 mm particles, in 1:1–5:1 solid/liquid ratio) for 30 min contact time at 25 °C
Conclusion
Using agricultural waste as a substrate for A. nidulans growth, such as wheat bran and tea waste, provides a superior alternative to the commonly used culture media, with superior growth and performance. The optimal growth and citric acid production of A. nidulans across different media compositions and process parameters. Notably, wheat bran and tea waste served as cost-effective substrates for the synthesis of high yield citric acid. Bioleaching of low-grade uranium ores with the metabolic products of A. nidulans is a promising and environmentally benign technique. Sucrose (100 g/L), peptone and yeast extract at 25 °C were the optimal carbon and nitrogen sources for maximum uranium extraction driven by citric acid. Rapid uranium extraction that reached 94% within 30 min was achieved from fine ore particles (0.075 mm) in a molar ratio of 2:1 (ore particle and fungal filtrate) highlighting the efficiency of the bioleaching process. In contrast to conventional acid leaching techniques, bioleaching by A. nidulans metabolite selectively recovered uranium without co-dissolving iron impurities. The amorphous nature of iron causes it to dissolve and precipitate with leached uranium, thereby inhibiting the leaching of pure uranium and reducing its purity. This represents the first application of uranium bioleaching that dissolves uranium without dissolving any associated uranium impurities. The economic viability and environmentally benign nature of this biotechnology highlights its potential to supplant conventional approaches. This innovative research developed a novel biological technique for uranium extraction from low-grade ores. The use of citric acid solutions derived from microorganisms for bioleaching has promising implications for environmentally responsible mining practices. Additional testing at the pilot scale can facilitate the industrial adoption of this revolutionary bioprocessing technology.
Data availability
The datasets generated and/or analyzed during the current study are present in the current manuscript.
Abbreviations
- C:
-
Carbon
- CA:
-
Citric acid
- LOI:
-
Loss on ignition
- TW:
-
Tea waste
- U:
-
Uranium
- WB:
-
Wheat bran
References
Abdel-Monem AA, El Aassy IE, El-Naggar AM, Attia KE, El-Fawy AG (1996) Concentrations of radon gas and daughters in uranium exploration tunnels, Allouga, West Central Sinai. Egypt Radiat Phys Chem 47:765–767. https://doi.org/10.1016/0969-806X(95)00173-U
Abostate MA, Saleh Y, Mira H, Amin M, Al Kazindar M, Ahmed BM (2018) Characterization, kinetics and thermodynamics of biosynthesized uranium nanoparticles (UNPs). Artif Cells Nanomed Biotechnol 46:147–159. https://doi.org/10.1080/21691401.2017.1301460
Abozaid SM, Shetaia YM, Rabie KA, Ahmed BM, Soliman ERS (2021) Recovery of uranium from solutions using Aspergillus nidulans isolated from monazite mineral. Int J Environ Anal Chem. https://doi.org/10.1080/03067319.2021.1939022
Amenaghawon NA, Aisien FA (2012) Modelling and simulation of citric acid production from corn starch hydrolysate using Aspergillus niger. Environ Nat Resour 2(1):73–85. https://doi.org/10.5539/ENRR.V2N1P73
AOAC (2002) Official method of analysis, 16th edn. Association of Official Analytical, Washington DC
Arzumanov TE, Shishkanova NV, Finogenova TV (2000) Biosynthesis of citric acid by Yarrowia lipolytica repeat-batch culture on ethanol. Appl Microbiol Biotechnol 53:525–529. https://doi.org/10.1007/S002530051651
Behera BC, Mishra R, Mohapatra S (2021) Microbial citric acid: production, properties, application, and future perspectives. Food Front 2:62–76. https://doi.org/10.1002/FFT2.66
Chergui D, Akretche-Kelfat S, Lamoudi L, Al-Rshaidat M, Boudjelal F, Ait-Amar H (2021) Optimization of citric acid production by Aspergillus niger using two downgraded Algerian date varieties. Saudi J Biol Sci 28:7134–7141. https://doi.org/10.1016/J.SJBS.2021.08.013
Das S, NaikDeshavath N, Goud VV, Dasu V (2019) Bioleaching of Al from spent fluid catalytic cracking catalyst using Aspergillus species. Biotechnol Rep 23:e00349. https://doi.org/10.1016/J.BTRE.2019.E00349
Davies W, Gray W (1964) A rapid and specific titrimetric method for the precise determination of uranium using iron (II) sulphate as reductant. Talanta 11:1203–1211. https://doi.org/10.1016/0039-9140(64)80171-5
DawnayStJ AB, Hirst AD, Perry DE, Chambers RE (1991) A critical assessment of current analytical methods for the routine assay of serum total protein and recommendations for their improvement. Ann Clin Biochem 28:556–567. https://doi.org/10.1177/000456329102800604
Del Campo G, Berregi I, Caracena R, Santos JI (2006) Quantitative analysis of malic and citric acids in fruit juices using proton nuclear magnetic resonance spectroscopy. Anal Chim Acta 556:462–468. https://doi.org/10.1016/J.ACA.2005.09.039
Dublet G, Lezama Pacheco J, Bargar JR, Fendorf S, Kumar N, Lowry GV, Brown GE (2017) Partitioning of uranyl between ferrihydrite and humic substances at acidic and circum-neutral pH. Geochim Cosmochim Acta 215:122–140. https://doi.org/10.1016/J.GCA.2017.07.013
Dusengemungu L, Kasali G, Gwanama C, Mubemba B (2021) Overview of fungal bioleaching of metals. Environ Adv 5:100083. https://doi.org/10.1016/J.ENVADV.2021.100083
El-Bondkly AMA, El-Gendy MMAA (2022) Bioremoval of some heavy metals from aqueous solutions by two different indigenous fungi Aspergillus sp. AHM69 and Penicillium sp. AHM96 isolated from petroleum refining wastewater. Heliyon https://doi.org/10.1016/J.HELIYON.2022.E09854
Gerayeli F, Ghojavand F, Mousavi SM, Yaghmaei S, Amiri F (2013) Screening and optimization of effective parameters in biological extraction of heavy metals from refinery spent catalysts using a thermophilic bacterium. Sep Purif Technol 118:151–161. https://doi.org/10.1016/J.SEPPUR.2013.06.033
Gharieb MM, Gadd GM (1999) Influence of nitrogen source on the solubilization of natural gypsum (CaSO4. 2H2O) and the formation of calcium oxalate by different oxalic and citric acid-producing fungi. Mycol Res 103:473–481. https://doi.org/10.1017/S0953756298007382
Gregor A, Kostrzewska E, Godorowska W (1977) Determination of serum proteins in the presence of dextran by means of the Biuret reaction. Infusionsther Klin Ernahr 4:48–50. https://doi.org/10.1159/000219790
Guinea J, Peláez T, Alcalá L, Bouza E (2005) Evaluation of Czapeck agar and Sabouraud dextrose agar for the culture of airborne Aspergillus conidia. Diagn Microbiol Infect Dis 53:333–334. https://doi.org/10.1016/J.DIAGMICROBIO.2005.07.002
Krishnamoorthy S, Ramakrishnan G, Dhandapani B (2021) Recovery of valuable metals from waste printed circuit boards using organic acids synthesized by Aspergillus niveus. IET Nanobiotechnol 15:212–220. https://doi.org/10.1049/NBT2.12001
Max B, Salgado JM, Rodríguez N, Cortés S, Converti A, Domínguez JM (2010) Biotechnological production of citric acid. Braz J Microbiol 41:862–875. https://doi.org/10.1590/S1517-83822010000400005
Mezaguer M, Kamel NEH, Lounici H, Kamel Z (2013) Characterization and properties of Pleurotus mutilus fungal biomass as adsorbent of the removal of uranium (VI) from uranium leachate. J Radioanal Nucl Chem 295:393–403. https://doi.org/10.1007/s10967-012-1911-y
Mostafa YS, Alamri SA (2012) Optimization of date syrup for enhancement of the production of citric acid using immobilized cells of Aspergillus niger. Saudi J Biol Sci 19:241–246. https://doi.org/10.1016/J.SJBS.2012.01.004
Narayanamurthy G, Ramachandra Y, Rai SP, Manohara YN, Kavitha BT (2008) Areca husk: an inexpensive substrate for citric acid production by Aspergillus niger under solid state fermentation. Indian J Biotechnol 7:99–102
Nuclear Energy Agency (NEA) (2014) NEA Annual Report. https://oecd-nea.org/jcms/pl_14936/2014-nea-annual-report
Pomraning KR, Bredeweg EL, Baker SE (2017) Regulation of nitrogen metabolism by GATA zinc finger transcription factors in Yarrowia lipolytica. mSphere https://doi.org/10.1128/MSPHERE.00038-17
Rasoulnia P, Barthen R, Lakaniemi AM (2021) A critical review of bioleaching of rare earth elements: the mechanisms and effect of process parameters. Crit Rev Environ Sci Technol 51:378–427. https://doi.org/10.1080/10643389.2020.1727718
Reddy MS, Kanwal HK (2022) Influence of carbon, nitrogen sources, inducers, and substrates on lignocellulolytic enzyme activities of Morchella spongiola. J Agric Food Res 7:100271. https://doi.org/10.1016/J.JAFR.2022.100271
Sati SC, Bisht S (2006) Utilization of various carbon sources for the growth of waterborne conidial fungi. Mycol 98:678–681. https://doi.org/10.3852/MYCOLOGIA.98.5.678
Sawant O, Mahale S, Ramchandran V, Nagaraj G, Bankar AV (2018) Fungal citric acid production using waste materials: a mini-review. J Microbiol Biotechnol Food Sci 8:821–828
Shah SS, Palmieri MC, Sponchiado SRP, Bevilaqua D (2020) Environmentally sustainable and cost-effective bioleaching of aluminum from low-grade bauxite ore using marine-derived Aspergillus niger. Hydrometallurgy 195:105368. https://doi.org/10.1016/j.hydromet.2020.105368
Shankar T, Sivakumar T (2016) Optimization of citric acid production using Aspergillus niger isolated from the leaf litter soil of Sathuragiri hills. Univers J Microbiol Res 4:79–87
Shapiro L, Brannock WW (1962) Rapid analysis of silicates carbonates and phosphates rocks. Geological Survey Bulletin. 114A
Sun J, Li G, Li Q, Yongdong W, Jianhong M, Caiyan P, Jing M (2020) Impacts of operational parameters on the morphological structure and uranium bioleaching performance of bio-ore pellets in one-step bioleaching by Aspergillus niger. Hydrometallurgy 195:105378. https://doi.org/10.1016/j.hydromet.2020.105378
Wang YD, Li GY, Ding DX, Zhang Z, Chen J, Hu N, Li L (2015) Column leaching of uranium ore with fungal metabolic products and uranium recovery by ion exchange. J Radioanal Nucl Chem 304:1139–1144. https://doi.org/10.1007/s10967-015-3957-0
Xu L, Yang H, Liu Y, Zhou Y (2019) Uranium leaching using citric acid and oxalic acid. J Radioanal Nucl Chem 321:815–822. https://doi.org/10.1007/s10967-019-06673-9
Zhou Z, Yang Z, Sun Z, Chen G, Xu L, Liao Q (2019) Optimization of bioleaching high-fluorine and low-sulfur uranium ore by response surface method. J Radioanal Nucl Chem 322:781–790. https://doi.org/10.1007/s10967-019-06712-5
Acknowledgements
The authors acknowledge with thanks The Nuclear Materials Authority.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
BMA contributed to Conceptualization, Formal analysis Methodology, Visualization, Writing original draft. AAM contributed to Formal analysis, Data curation, Methodology and Writing original draft. NAK contributed to Data curation, Methodology. IEE contributed to Conceptualization, Investigation, Visualization, ERSS contributed to Investigation, Methodology, Data curation, Writing the published version of the manuscript and visualize the data. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Editorial responsibility: Bivin Thomas.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ahmed, B.M., Mohammed, A.A., Kawady, N.A. et al. Costly effective bioleaching of valuable metals from low grade ore using Aspergillus nidulans. Int. J. Environ. Sci. Technol. 21, 5469–5482 (2024). https://doi.org/10.1007/s13762-023-05355-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-023-05355-0