Abstract
Conventional chemical coagulants are used for potable water treatment, which requires unsustainable mining and transformation of raw materials for their production with costly sludge disposal. Natural coagulants are potential alternatives to chemical coagulants. This study emphasized the use of natural eco-friendly coagulants (oat and onion seed) for turbid water treatment. Coagulant seeds were characterized by different techniques such as SEM, EDX, FTIR, and zeta potential analyzer. Response surface method (RSM) was used to optimize the coagulation process. The effect of initial water turbidity, pH, coagulant seed extract dose, slow mix time, and settling time on the efficiency of turbidity removal was studied. The relation between removal efficiency and the main three significant operational parameters (initial water turbidity, pH, and coagulant extract dose) was fitted to a quadratic model. The optimal removal was achieved at alkaline and neutral media (pH = 7–11 for oat extract and pH = 7–9 for onion extract). The turbidity removal efficiency was 99% using 6 ml/L oat extract dose and 98.9% using 4 ml/L onion extract dose at pH equal to 8 and initial turbidity of 190 NTU. The effect of using oat extract or onion extract with alum was studied, and the results indicated that the optimum dose was found to be 1 ml/L of a natural extract with 10 mg/L alum. Furthermore, oat and onion extract aids were able to decrease the organic load in the treated water. Oat and onion seed extracts were proven to be efficient and eco-friendly natural coagulants or coagulant aids for surface water treatment.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Clean and safe water access is a major concern worldwide. Suitable water treatment is necessary to remove impurities, turbidity, and other pathogenic bacteria. The coagulation/flocculation process is one of the most vital processes in water treatment. There are various coagulants used for water coagulation, chemical coagulants such as ferric and aluminum compounds, and they are the most common coagulants used due to cost-effective (Ahmad et al. 2022). The use of chemical coagulants affects human health and causes severe problems, especially in the stage of sludge treatment and disposal. Aluminum compounds have potential links to Alzheimer’s disease and cancer (Rondeau et al. 2000; Bodlund et al. 2013). Moreover, using chemical coagulants produces large amounts of sludge (Divakaran and Pillai 2002). In addition, synthetic organic polymers have carcinogenic and neurotoxic effects (Fenouille et al. 2012). Recently, there has been extensive interest in developing eco-friendly natural coagulants which can be extracted from plants, animals, or microorganisms tissues due to their positive effect on the environment and the ecosystem comparing to chemical coagulants; moreover, they do not change the treated water pH (Saleem and Bachmann 2019; Šćiban et al. 2009). In addition, natural coagulants are cost-effective, non-toxic, renewable and can efficiently remove turbidity, while chemical coagulants are effective in a defined range of pH and change the treated water pH (Alazaiza et al. 2022; Koul et al. 2022). Using plants as a green method for extracting natural coagulants is more common in addition to other purposes (Hashem et al. 2021).
Various natural materials have been investigated as a coagulant sources such as Phaseolus vulgaris (Antov et al. 2010; Muthuraman and Sasikala 2014), Quercus robur (Antov et al. 2018), local plant leaves (Ahmad et al. 2022), Maerua subcordata and Moringa stenopetala (Megersa et al. 2019), Pine cone (S. Hussain et al. 2019), Opuntia stricta (G. Hussain and Haydar 2021), Opuntia ficus-indica (Wan et al. 2019), walnut seed extract (Zedan et al. 2022), Plantago ovata (Ramavandi 2014), acorn and chestnut (Šćiban et al. 2009), chitosan (Mohammad et al. 2018), and Moringa oleifera (Baptista et al. 2017; Ali et al. 2010; Liew et al. 2006; Garde et al. 2017; Dotto et al. 2019; Gaikwad and Munavalli 2019). In addition, three natural coagulants from moringa oleifera seed (seed husk, ground seed, and degreased) were extracted for the treatment of greywater (Peña-Guzmán, C., & Ortiz-Gutierrez 2022). Moreover, fenugreek as a coagulant, with Aloe vera and okra as flocculants, were studied to treat the effluent water of palm oil mill (Lim et al. 2021). Combining natural coagulants with functionalized nanoparticles was also studied as an effective alternative in surface water treatment such as magnetite nanoparticles with coagulant extracted from the Moringa oleifera (Mateus et al. 2018). Furthermore, the improvement of electrocoagulation–electroflotation process by a natural coagulant derived from Opuntia ficus-indica was investigated (Adjeroud-abdellatif et al. 2020). A recent study using a composite of cactus-banana peels as a natural coagulant for turbidity removal showed that the optimum cactus-to-banana ratio was 0.62:0.38 (Mpagi et al. 2023). Also, it was observed that using Annona diversifolia seed extract as a natural coagulant works well in acidic conditions for water treatment (Muntaqa et al. 2023).
Modeling and optimization are of great concern in any process. Modeling and optimization using a single variable method does not present the interaction between different variables in the process and is outdated. Response surface method (RSM) is a statistical optimization technique that contains experimental design, analysis, and modeling through building multivariate equations, evaluating their optimal values, and showing the effects of multiple factors and the interaction between their effects (Adedayo et al. 2019; Teh et al. 2014). RSM has been applied in different processes of water and wastewater treatment but there is no such research on coagulation of surface water using oat or onion seed extract. Moreover, to use natural materials as a source of coagulant, it is essential to find easily achievable and locally available materials. So, the aim of this study is to optimize the coagulation process using novel natural coagulants extracted from oat seed and onion seed. To minimize the surface water turbidity, the response surface method was applied to determine the effects of three main operating parameters (initial turbidity, coagulant extract dose, and pH) on the performance of the coagulation process. Additionally, using oat and onion extracts as coagulant aids with alum was investigated.
Materials and methods
Coagulants extract preparation
Oat and onion seeds were obtained from Mansura City—Egypt. The seeds were ground with a mortar and pestle and then with a laboratory mill to obtain a fine powder. Separately, five grams of each powder was stirred with 100 mL of NaCl solution with 1 molar concentration at 550 rpm for 30 h using a magnetic stirrer (JSHS-180. digital hotplate stirrer, Korea) at room temperature 25 °C to achieve best dissolving and extract the coagulation active components (Antov et al. 2018). The suspension was gravity filtered through a tough filter paper, then through a 45 µm filter after first passing through a piece of gauze. For further use, the rude extracts, filtered solutions, were stored in glass vials and kept in a refrigerator at 4 °C.
Synthetic turbid water preparation
Synthetic turbid water was prepared for coagulation tests by adding 15 g of dried kaolin in 1 L of tap water discharged from the river Nile. The average total organic carbon (TOC) and initial turbidity of the tap water were 42 mg/L and 5 NTU, respectively. In addition, the TDS and chemical properties of the tap water are shown in Table 1. The kaolin and water were stirred at 120 rpm for 2 h to achieve a uniform suspension of kaolin particles. The suspension remained for 24 h to complete the particles hydration (Okuda et al. 2001). This procedure is conducted to control the properties of water and obtain different levels of turbidity. Before the coagulation experiments, suspensions with desired initial turbidity were prepared by adding consecutive dilutions just before the coagulation test. The pH of the synthetic water was initially adjusted to the desirable range from 3 to 11 using HCl or NaOH (1 M). A pH meter (EXTECH instruments 341350A, PH/Conductivity, TDS/Salinity/ORP Meter) was used to measure the solution pH.
Coagulant characterization
Oat and onion seed powder were characterized using various techniques. The surface morphology of oat and onion powder samples was inspected using a scanning electron microscope (SEM) (JEOL JSM 6510 lv, Japan) which gives a high-quality and clear stereoscopic image. Energy-dispersive X-ray (EDX) (Oxford X-MaxN 20, USA) was used to examine the compositions of the samples. The Fourier-transform infrared spectroscopy (FTIR) (Nicolet™ IS 10 FTIR) spectrometer was used to reveal the functional groups in the surface of the samples with a spectral range from 400 to 4000 cm−1. Furthermore, the surface charge of the samples was analyzed using zeta potential analyzer (Malvern Zeta size Nano-zs90).
Coagulation experiments
The coagulation activity of oat and onion seed extracts was evaluated using Jar test experiments. Using six beakers of 1000 mL capacity, 500 ml of desired turbid water was filled in beakers. Oat and onion extract with various doses were added to beakers and agitated rapidly for 5 min at 140 rpm then stirred gently for 30 min (except in slow mixing time effect) at 40 rpm. After that, solutions in beakers were left to settle for 1 h (except in the settling time effect). The residual turbidity for samples collected from the top of beakers was measured using a turbid meter (TB300 IR Turbid meter). The experiments were implemented at an ambient temperature 25 ± 2 °C. The effect of operating parameters as pH (3–11), coagulant dose (2–6 l/L), initial turbidity (10–200 NTU), slow mixing time (10–35 min), and settling time (15–90 min) was investigated. A blank sample was prepared without any coagulant addition and used in the jar test. The final turbidity of the blank was measured after settling to determine the coagulation activity of extracts. The efficiency and activity of coagulants were calculated by Eqs. (1) and (2) (Yan et al. 2016).
Turbidity measurements after and before treatment represent, respectively, the initial and final turbidity of the sample. Moreover, to examine the coagulant extract from oat and onion seeds as coagulant aids, the effect of using the extract with alum was tested, and 10 mg/L of alum dose was stirred combined with various coagulant extract doses from 0 to 2 ml/L at pH 8 with initial turbidity of 50 NTU for 5 min at 140 rpm then for 30 min at 30 rpm then keep settling for 30 min. The final turbidity of samples was measured after the 30 min settling time.
The chemical analysis and organic load of treated water
The chemical analysis and TDS of water after using oat and onion extract were tested to investigate the effect of using those natural coagulants on the chemical properties of the treated water. The organic matter in the treated water was increased after using some natural coagulants (Camacho et al. 2017). Within this work, total organic carbon (TOC) in raw water and water after the coagulation process using oat and onion natural coagulant was measured to assess the organic load increase. Six beakers of 1 L were filled with raw water (initial turbidity of 50 NTU and TOC = 42 mg/L). Various doses of coagulant extract (oat and/or onion) varied from 2 to 6 ml/L were added to the beakers at pH 8 to examine the impact of using oat and onion extract on the organic load. Moreover, a blank turbid sample without any coagulant addition was prepared. The final TOC value of the blank and for each beaker was measured after 30 min of settling using AJ-Analyzer multi-N/C 3100. Furthermore, the TOC value was examined after using alum and compared to using oat and onion extract separately or combined with alum.
Experimental design
Response surface methodology (RSM) was used to optimize the parameters affecting the coagulation process as the response. For each coagulant, twenty runs of experiments were produced using Box–Behnken factorial design. The initial turbidity (NTU) (X1), coagulant dose (X2), and pH (X3) were selected as three independent parameters during the preparation process. The range and levels of the parameters are listed in Table 2. The turbidity removal efficiency was selected as the dependent variable. An equation based on a quadratic polynomial of second order was used to fit the response variable:
where Y (%) is the turbidity removal efficiency after 60 min of sedimentation, Xi and Xj are the independent factors, βo, β1, β2, and βij are the coefficients of the quadratic equation, ε is the residual term Eq. (3), and n represents the number of dependent parameters. Minitab© 18 software and analysis of variance (ANOVA) were used to perform the regression analysis and significance of the model, respectively (Shayegan et al. 2013). The optimum values of operational parameters were concluded by defining the optimum removal efficiency of turbidity as a target after 60 min of sedimentation.
Results and discussion
Coagulant characterization
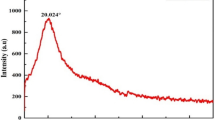
Figure 1a–d shows the SEM micrographs obtained from the oat and onion powder. The micrographs show the existence of platelet networks with porous and rough surfaces which affect the coagulation/flocculation process. Porous and rough surfaces improve the linking mechanism due to the presence of a large surface area which provides more adsorption sites (Shak and Wu 2015). Figure 1e–f shows the EDX of the oat and onion powder. The EDX analysis showed that oat samples contain different elements such as C, K, O, P, S, Zn, and Cu, while the EDX analysis of onion seed powder revealed the existence of elements such as C, O, Mg, Si, P, S, K, Ca, and Cu as shown in Table 3. This shows the existence of active elements for both coagulants that boost the removal’s effectiveness (Mateus et al. 2018).
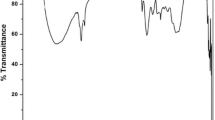
FTIR spectra for coagulants were taken at wave number ranged from 400 to 4000 cm−1 as shown in Fig. 2a. O–H stretching originated in the fatty acids, protein, carbohydrates, and lignin is ascribed by the wide band at 3418 and band at 3906 cm−1 for oat and the wide band at 3449 cm−1 and bands from 3906 to 3714 cm−1 for onion (Younes et al. 2021, 2019). The peaks at 2857 and 2928 cm−1 for oat and peaks at 2865 and 2926 cm−1 for onion are attributed to C–H group (N. El-Bendary et al. 2021a, b; Fawzy et al.2022), while the peaks of 1654 and 1744 cm−1 of oat and peaks from 1655 to 1849 cm−1 of onion are assigned to COO– double bond of deprotonated carboxylate groups (Kakoi et al. 2016). The oat peak of 1544 cm−1 and onion peak of 1546 cm−1 indicated the presence of the C–N elongation vibration (Mateus et al. 2018). Bands from 1243 to 1459 cm−1 of oat and from 1243 to 1460 cm−1 of onion could be associated with C–O stretching (El-Bendary et al. 2021a, b). The bands from 1020 to 1156 cm−1 and bands from 1027 to 1164 cm−1 are assigned to the C–OH hydroxyl bond (Mateus et al. 2018) for oat and onion, respectively. The band of oat at 927 cm−1 is ascribed to C–O–C skeletal vibration. Moreover, the characteristic peaks of CH2 deformation were observed for oat and onion at bands from 710 to 860 cm−1 and from 722 to 875 cm−1, respectively (Yan et al. 2016). In addition, the peaks from 529 to 611 cm−1 for oat and from 422 to 613 cm−1 for onion correspond to metal oxygen and hydroxyl (Hani Mahanna and Azab 2020; H. Mahanna and El-Bendary 2022). Thus, the FTIR spectra of oat coagulant showed the presence of different functional groups that are actively involved in the formation of flocs and turbidity removal.
Figure 2b shows the zeta potential of oat and onion coagulants in terms of pH. The zeta potential of oat and onion coagulants at pH 2.0 was + 2.44 mV and + 2.52 mV and sharply decreased to − 13.36 mV and − 5.48 mV at pH 10, respectively. The point of zero charge of natural coagulants would be between pH 3.0 and 4.0. As the earlier FTIR illustration, the proteins with hydroxyl and carboxyl groups on the coagulant surface are associated with the negative charge potential. (Yan et al. 2016). The higher intensity charge variation for coagulant extracts suggests that the pH variation led to a greater number of dissociable groups (Mateus et al. 2018). The higher zeta potential value indicated the higher performance in the coagulation activity (Takaoka et al. 2017).
Optimization of turbidity removal efficiency
The details of the experimental data for the observed and predicted values of removal efficiency (R%) are shown in Table 4 for oat extract and Table 5 for onion extract. A second-order polynomial equation expresses the relation between the variables and the response as shown by Eq. (4) for oat and Eq. (5) for onion.
where R (%) is the turbidity removal efficiency ratio after 60 min of sedimentation, X1 (NTU), X2 (dose), and X3 (pH) represent initial turbidity in NTU, oat extract dose in ml/L, and pH, respectively. The analysis of variance (ANOVA) shown in Tables S1 and S2 confirms the significance and adequacy of the models. Furthermore, small P values and high F-values ensured the significance of the models. Corresponding to F-values, the effect of factor values on the turbidity removal efficiency can be ordered as NTU > pH > dose for both coagulants as shown in Tables S1 and S2 in supplementary materials.
The three-dimensional and contour plots in Fig. 3 clarify the effect of the initial turbidity concentration, coagulant extract dose, and pH on the efficiency of turbidity removal. The response surface plots provide a visual tool for the prediction of the turbidity removal efficiency by different values of the applied parameters, and the contour plots help to identify the interactions between these parameters (Adedayo et al. 2019). The interaction effects between initial turbidity vs coagulant extract dose, initial turbidity vs. pH, and coagulant extract dose vs pH on turbidity removal efficiency are presented in Fig. 3a for oat coagulant and in Fig. 3b for onion coagulant. For both coagulants, turbidity removal efficiency R (%) decreased with decreasing initial turbidity, decreasing coagulant extract dose, and decreasing pH and the dose factor was of low influence on the removal efficiency as shown in Fig. 3a, b. The percentage of R (%) remains low despite changing the value of oat dose in case of low pH and the removal efficiency increased with increasing pH and oat dose together or increasing pH and initial turbidity together as shown in Fig. 3a. The percentage of R (%) remains low despite changing the value of onion dose in case of low initial turbidity and the removal efficiency increased with increasing initial turbidity and onion dose together or increasing initial turbidity and pH together as shown in Fig. 3b.
Effect of operational parameters
Effect of coagulant extract dose and initial turbidity
Turbidity removal efficiency and coagulation activity of oat and onion natural coagulants at different initial turbidity (10, 50, and 190 NTU) were investigated at various doses as shown in Fig. 4. The results in Fig. 4a, b suggest a certain effect of initial turbidity on coagulation activity. It is obvious that with an increase in initial turbidity from 10 to 190 NTU, the coagulation activity increased from 66 to 97% at an oat dose of 6 ml/L, while the coagulation activity increased from 76.2 to 97.5% at onion dose of 4 ml/L. Furthermore, by increasing initial turbidity from 10 to 190 NTU, the turbidity removal efficiency increased from 78 to 99% at 6 ml/L oat dose and from 86.5 to 98.9% at 4 ml/L onion dose as shown in Fig. 4c, d. This is due to that the dense presence of particles in water increases the possibility of collision and the formation of dense flocs and increases the sedimentation process and thus increases the removal efficiency (Camacho et al. 2017). The oat extract dose up to 3 ml/L and onion extract dose up to 2 ml/L significantly affected the coagulation activity in Fig. 4a, b. At initial turbidity of 190 NTU, the coagulation activity obviously increased significantly using oat dose of up to 3 ml/L and onion dose of up to 2 ml/L. By using oat dose from 3 to 6 ml/L and onion dose from 2 to 4 ml/L, the coagulation activity was slightly increased to reach 97% with 6 ml/L oat dose and 97.5% with 4 ml/L onion dose. This is due to increasing the number of active compounds with coagulation potential (Ang and Mohammad 2020). It has been noticed that the efficiency of turbidity removal of coagulants extracted from oat and onion was similar to efficiencies of some previously investigated natural materials such as Mustard seed extracts (Bodlund et al. 2014) and Moringa oleifera (Ghebremichael et al. 2006).
Effect of pH
The pH is a vital factor in the coagulation process where it can adjust the pollutant or coagulant surface charge (Daverey et al. 2019; Norfazilah et al. 2022). Thus, the effect of solution pH (3.0 to 11.0) on turbidity removal by oat and onion extracts was studied at initial turbidity of 60 and 190 NTU as shown in Fig. 5. With increasing pH from 3 to 11 for oat extract coagulant, it was shown that the turbidity removal efficiency increased from 92 to 97% and from 97 to 99.2% at 60 and 190 NTU, respectively. The higher removal efficiency was noticed at pH 11. This may be due to the cationic protein components that are more activated at higher pH values (S. Hussain et al. 2019). The highest removal efficiency achieved at pH 7 by onion seed extract was 96.4 and 97.5% for 60 and 190 NTU, respectively. So, neutral and higher pH values achieve the best turbidity removal. So, the results indicate that the natural coagulant from oat and onion seed extract was highly efficient at a broad range of pH and can be applied as a coagulant.
Effect of slow mix and settling time
Figure 6a shows the effect of slow mixing time on the turbidity removal. Slow mixing time has an active influence, where increasing slow mixing time increases the possibility of extract coagulating and making flocs with a suitable size for faster sedimentation. By increasing the slow mix time from 10 to 20 min, the turbidity removal efficiency increased gradually from 84 to 94% and from 93.3 to 95.2% by oat and onion extracts, respectively, at initial turbidity of 60 NTU and 60 min settling time. There is an obvious increase in the removal efficiency using oat extract and an insignificant increase in the removal efficiency using onion extract, while the effect starts to diminish at a slow mix time from 20 to 30 min for both coagulants. Slight performance was noticed beyond the slow mix duration of 30 min due to the re-stabilization of flocs (Subramonian et al. 2014).
Furthermore, settling time is a considerable factor in the performance of the removal efficiency. The effect of settling time on the removal efficiency is shown in Fig. 6b at a pH of 7.8, using initial turbidity of 70 and 195 NTU, and oat and onion extract dose of 6 and 4 ml/L, respectively. After 30 min of settling time using oat extract coagulant, the removal efficiency was 93.6, and 98.5% at initial turbidity of 70, and 195 NTU, respectively, while after 60 min of settling time, the removal efficiency was 96.8, and 99.1% at initial turbidity 70, and 195 NTU, respectively. After 30 min of settling time using onion extract coagulant, the removal efficiency was 95, and 97.8% at initial turbidity of 70, and 195 NTU, respectively, while after 60 min of settling time, the removal efficiency was 97.5, and 99.0% at initial turbidity 70, and 195 NTU, respectively. As shown in Fig. 6b, there is an insignificant effect on the removal efficiency after a settling time of more than 60 min for both coagulants, while, at settling time from 30 to 60 min, the effect on the removal efficiency was obviously exhibited especially at low turbidity compared to high turbidity. This may be due to the presence of active protein in the extract dose with high rates of reaction within 30–60 min of settling according to initial turbidity and then gradually declines with time (Zedan et al. 2022; Shak and Wu 2014).
Effect of using extracts as coagulant aids
The use of oat and onion extract coagulant as a coagulant aid in combination with alum was investigated. Therefore, the effect of using natural coagulant in combination with alum was studied at an initial turbidity of 50 NTU, settling time 30 min, and pH 8. Figure 7a shows the removal efficiency at different alum doses ranging from 5 to 25 mg/L. It was shown that the removal efficiency increased with increasing the alum dose from 5 to 20 mg/L and then the removal efficiency decreased using an alum dose greater than 20 mg/L. The highest removal efficiency of 91% was achieved using alum only with a dose of 20 mg/L. Using a dose of 10 mg/L of alum only, the turbidity removal efficiency reached 78% while by adding 0.75 ml/L of oat and onion extract to 10 mg/L of alum the removal efficiency increased to 92 and 95.8%, respectively, as shown in Fig. 7b. The highest removal efficiency reached 93 and 96% using oat and onion extract doses of 1.0 ml/L combined with an alum dose of 10 mg/L, respectively. It was observed that using oat and onion extract as a coagulant aid was proficient in enhancing the performance of coagulation activity. Besides, it can be applied to reduce the required alum dose for the coagulation process. Furthermore, the volume of sludge accumulated from different types and doses of coagulants was measured. The results of sludge volume per one liter for an initial turbidity of 100 NTU are shown in Fig. 7c. It was observed that using 6 ml/L of oat extract and 4 ml/L of onion extract gives the highest sludge volume compared to other coagulants which confirmed that the highest removal efficiency was obtained by those doses.
The chemical analysis and organic load of coagulated water
The chemical analysis of coagulated water after using the oat and onion coagulant extract (optimum conditions) is shown in Table 6. To examine the effect of using oat and onion coagulant extract on the organic load after coagulation, the total organic carbon (TOC) in water was analyzed. Figure 8a shows the effect of using the extract dose on the TOC values after the coagulation process at initial turbidity of 50 NTU and pH 8. It was shown that after 30 min of settling, the TOC of the blank was 38.5 mg/L due to gravity settling of some organic suspended particles. It was noticed that the TOC values decreased from 39.48 to 37.5 mg/L and from 39.2 to 35.89 mg/L by increasing of dose from 2 to 6 ml/L for oat and onion, respectively. This means that increasing the coagulant extract dose has no significant effect on the organic matter concentration. Moreover, the values of TOC at different coagulant doses are within the required limits (RS2 2012). Moreover, the organic content was also examined for coagulant extract combined with alum. Figure 8b shows the TOC values after the coagulation process using different coagulants at initial turbidity of 50 NTU, pH 8, and settling time 30 min. The results showed that the TOC was 38, 36.75, 38.9, 37.5, and 38.1 mg/L using 6 ml/L oat only, 4 ml/L onion only, 2 ml/L oat with 10 mg/L alum, 2 ml/L onion with 10 mg/L alum, and 10 mg/L alum only, respectively, while the TOC value of the blank beaker after 30 min of settling was 38.5 mg/L. This means that using oat or onion extract combined with alum has no significant impact on the organic matter. This revealed that using oat and onion extract as coagulants has an insignificant impact on the TDS and chemical properties of coagulated water.
Comparison with several natural coagulants
In this study, oat seed extract and onion seed extract were used as eco-friendly coagulants for turbidity removal from water. These extracts, either alone or in combination with alum, were proven to be efficient at removing turbidity. Table S3 shows a comparison between the oat extract and onion extract coagulants with different other natural coagulants. As indicated in Table S3, small doses of oat and/or onion seed extract give the highest removal efficacy compared to other natural coagulants for turbidity removal. At initial water turbidity ranged from 10 to 190 NTU, it was found that 6 ml/L oat extract and 4 ml/L onion extract give high turbidity removal efficiency ranging from 78 to 99% and from 86.5 to 98.5%, respectively. In addition, 1 ml/L oat extract with 10 mg/L alum and 1 ml/L onion extract with 10 mg/L alum give 93 and 96% removal efficiency, respectively. Oat extract and onion extract coagulants can therefore be employed as natural, eco-friendly coagulants or as coagulant aids with alum to remove turbidity from aqueous solutions.
Conclusion
The present study has proved the potential of oat seed extract and onion seed extract to be used as natural coagulants for water turbidity removal. A response surface method (RSM) was successfully applied to optimize the coagulation process. Moreover, FTIR, SEM, EDX, and zeta potential analyzer were applied in oat and onion seeds characterization. The experimental results showed that the turbidity removal efficiency reached 99% using an oat extract dose of 6 ml/L and 98.9% using onion extract dose of 4 ml/L at pH equal to 8 and initial turbidity of 190 NTU. Furthermore, neutral and basic pH values achieved high turbidity removal efficiency, and the highest removal was achieved at pH range from 7 to 11 and from 7 to 9 for oat extract and onion extract, respectively. The oat extract or onion extract can also be used as coagulant aids with alum with an optimum dose of 1 ml/L with 10 mg/L of alum. In addition, using the oat and onion extracts helps to reduce the organic content in treated water. Oat and onion extract coagulants have an insignificant impact on the TDS and chemical properties of coagulated water. Oat and/or onion seed extract gives the highest removal efficacy compared to other natural coagulants for turbidity removal. Oat and onion seed extracts can be considered efficient and eco-friendly natural main coagulants or coagulant aids for water turbidity removal.
Data availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
References
Adesina OA, Abdulkareem F, Yusuff AS, Lala M, Okewale A (2019) Response surface methodology approach to optimization of process parameter for coagulation process of surface water using Moringa oleifera seed. South Afr J Chem Eng 28:46–51. https://doi.org/10.1016/j.sajce.2019.02.002
Adjeroud-abdellatif N, Hammoui Y, Boudria A, Choulak F, Leclerc J-P, Merzouk B (2020) Effect of a natural coagulant extract from Opuntia ficus-indica cladode on electrocoagulation–electroflotation water treatment process. Int J Environ Anal Chem 00(00):1–25. https://doi.org/10.1080/03067319.2020.1804889
Ahmad A, Abdullah SRS, Hasan HA, Othman AR, Ismail NI (2022) Potential of local plant leaves as natural coagulant for turbidity removal. Environ Sci Pollut Res 29(2):2579–2587. https://doi.org/10.1007/s11356-021-15541-7
Alazaiza MY, Albahnasawi A, Ali GA, Bashir MJ, Nassani DE, Al Maskari T, Amr SS, Abujazar MS (2022) Application of natural coagulants for pharmaceutical removal from water and wastewater: a review. Water 14(2):1–16. https://doi.org/10.3390/w14020140
Ali EN, Muyibi SA, Salleh HM, Alam MZ, Mohd RM, Salleh. (2010) Production of natural coagulant from Moringa oleifera seed for application in treatment of low turbidity water. J Water Resour Prot 02(03):259–266. https://doi.org/10.4236/jwarp.2010.23030
Ang WL, Mohammad AW (2020) State of the art and sustainability of natural coagulants in water and wastewater treatment. J Clean Prod 262:121267. https://doi.org/10.1016/j.jclepro.2020.121267
Antov MG, Šćiban MB, Petrović NJ (2010) Proteins from common bean (Phaseolus vulgaris) seed as a natural coagulant for potential application in water turbidity removal. Biores Technol 101(7):2167–2172. https://doi.org/10.1016/j.biortech.2009.11.020
Antov MG, Šćiban MB, Prodanović JM, Kukić DV, Vasić VM, Đorđević TR, Milošević MM (2018) Common oak (Quercus robur) acorn as a source of natural coagulants for water turbidity removal. Ind Crops Prod 117:340–346. https://doi.org/10.1016/j.indcrop.2018.03.022
Baptista AT, Alves MO, Silva RG, Gomes RB, Vieira MF, Vieira AMS (2017) Protein fractionation of seeds of Moringa oleifera lam and its application in superficial water treatment. Sep Purif Technol 180:114–124. https://doi.org/10.1016/j.seppur.2017.02.040
Bodlund I, Pavankumar AR, Chelliah R, Kasi S, Sankaran K, Rajarao GK (2014) Coagulant proteins identified in mustard: a potential water treatment agent. Int J Environ Sci Technol 11(4):873–880. https://doi.org/10.1007/s13762-013-0282-4
Bodlund I, Sabarigrisan K, Chelliah R, Sankaran K, Rajarao GK (2013) Screening of coagulant proteins from plant material in southern India. Water Sci Technol Water Supply 13(6):1478–1485. https://doi.org/10.2166/ws.2013.156
Camacho FP, Sousa VS, Bergamasco R, Teixeira MR (2017) The use of Moringa oleifera as a natural coagulant in surface water treatment. Chem Eng J 313:226–237. https://doi.org/10.1016/j.cej.2016.12.031
Daverey A, Tiwari N, Dutta K (2019) Utilization of extracts of Musa paradisica (banana) peels and Dolichos lablab (Indian bean) seeds as low-cost natural coagulants for turbidity removal from water. Environ Sci Pollut Res 26(33):34177–34183. https://doi.org/10.1007/s11356-018-3850-9
Divakaran R, Sivasankara Pillai VN (2002) Flocculation of algae using chitosan. J Appl Phycol 14(5):419–422. https://doi.org/10.1023/A:1022137023257
Dotto J, Fagundes-Klen MR, Veit MT, Palácio SM, Bergamasco R (2019) Performance of different coagulants in the coagulation/flocculation process of textile wastewater. J Clean Prod 208:656–665. https://doi.org/10.1016/j.jclepro.2018.10.112
El-Bendary N, El-Etriby HK, Mahanna H (2021a) High-performance removal of iron from aqueous solution using modified activated carbon prepared from corn cobs and luffa sponge. Desalin Water Treat. https://doi.org/10.5004/dwt.2021.26721
El-Bendary N, El-Etriby HK, Mahanna H (2021b) Reuse of adsorption residuals for enhancing removal of ciprofloxacin from wastewater. Environ Technol. https://doi.org/10.1080/09593330.2021.1952310
Fawzy A, Mahanna H, Mossad M (2022) Effective photocatalytic degradation of amoxicillin using MIL-53(Al)/ZnO composite. Environ Sci Pollut Res 29(45):68532–68546. https://doi.org/10.1007/s11356-022-20527-0
Fenouille N, Tichet M, Dufies M, Pottier A, Mogha A, Soo JK, Rocchi S et al (2012) The epithelial-mesenchymal transition (EMT) regulatory factor SLUG (SNAI2) is a downstream target of SPARC and AKT in promoting melanoma cell invasion. PLoS ONE 7(7):e40378. https://doi.org/10.1371/journal.pone.0040378
Gaikwad VT, Munavalli GR (2019) Turbidity removal by conventional and ballasted coagulation with natural coagulants. Appl Water Sci 9(5):1–9. https://doi.org/10.1007/s13201-019-1009-6
Garde WK, Buchberger SG, Wendell D, Kupferle MJ (2017) Application of Moringa oleifera seed extract to treat coffee fermentation wastewater. J Hazard Mater 329:102–109. https://doi.org/10.1016/j.jhazmat.2017.01.006
Ghebremichael KA, Gunaratna KR, Dalhammar G (2006) Single-step ion exchange purification of the coagulant protein from Moringa oleifera seed. Appl Microbiol Biotechnol 70(5):526–532. https://doi.org/10.1007/s00253-005-0130-7
Hashem MA, Payel S, Hasan M, Momen MA, Sahen MS (2021) Green preservation of goatskin to deplete chloride from tannery wastewater. HighTech Innov J 2(2):99–107
Hussain G, Haydar S (2021) Textile effluent treatment using natural coagulant Opuntia stricta in comparison with alum. Clean: Soil, Air, Water 2000342:1–10. https://doi.org/10.1002/clen.202000342
Hussain S, Sattar A, Ahmad A (2019) Pine cone extract as natural coagulant for purification of turbid Water. Heliyon 5(3):e01420. https://doi.org/10.1016/j.heliyon.2019.e01420
Tijjani Usman IM, Lee FW, Ho YC, Khaw HP, Chong QW, Kee YM, Lim JW, Show PL (2023) Evaluation of annona diversifolia seed extract as a natural coagulant for water treatment. Sustainability 15(7):6324. https://doi.org/10.3390/su15076324
Kakoi B, Kaluli JW, Ndiba P, Thiongo G (2016) Banana pith as a natural coagulant for polluted river water. Ecol Eng 95:699–705. https://doi.org/10.1016/j.ecoleng.2016.07.001
Koul B, Bhat N, Abubakar M, Mishra M, Arukha AP, Yadav D (2022) Application of natural coagulants in water treatment: a sustainable alternative to chemicals. Water 14(22):3751. https://doi.org/10.3390/w14223751
Liew AG, Noor MJMM, Muyibi SA, Fugara AMS, Muhammed TA, Iyuke SE (2006) Surface water clarification using M. oleifera seeds. Int J Environ Stud 63(2):211–219. https://doi.org/10.1080/00207230500117670
Lim KS, Sethu V, Selvarajoo A (2021) Natural plant materials as coagulant and flocculants for the treatment of palm oil mill effluent. Mater Today Proc 48:871–887. https://doi.org/10.1016/j.matpr.2021.02.483
Mahanna H, El-Bendary N (2022) Enhanced catalytic oxidation of reactive dyes by reuse of adsorption residuals as a heterogeneous catalyst with persulfate/UV process. Int J Environ Sci Technol 19(11):10945–10956. https://doi.org/10.1007/s13762-021-03856-4
Mahanna H, Azab M (2020) Adsorption of reactive red 195 dye from industrial wastewater by dried soybean leaves modified with acetic acid. Desalin Water Treat 178:312–321. https://doi.org/10.5004/dwt.2020.24960
Mateus GA, Pisano MP, Paludo TR, Dos Santos T, Silva MF, Nishi L, Fagundes-Klen MR, Gomes RG, Bergamasco R (2018) Obtaining drinking water using a magnetic coagulant composed of magnetite nanoparticles functionalized with moringa oleifera seed extract. J Environ Chem Eng 6(4):4084–4092. https://doi.org/10.1016/j.jece.2018.05.050
Megersa M, Beyene A, Ambelu A, Triest L (2019) Coupling extracts of plant coagulants with solar disinfection showed a complete inactivation of faecal coliforms. Clean: Soil, Air, Water 47(1):1700450. https://doi.org/10.1002/clen.201700450
Mohammad M, Kahforoushan D, Abbasi F (2018) Using chitosan/CHPATC as coagulant to remove color and turbidity of industrial wastewater : optimization through RSM design. J Environ Manage 211:347–355. https://doi.org/10.1016/j.jenvman.2018.01.031
Mpagi H, Wilberforce P, Maxwell O (2023) Results in engineering synthesis and efficacy of cactus-banana peels composite as a natural coagulant for water treatment. Results Eng 17:100945. https://doi.org/10.1016/j.rineng.2023.100945
Muthuraman G, Sasikala S (2014) Removal of turbidity from drinking water using natural coagulants. J Ind Eng Chem 20(4):1727–1731. https://doi.org/10.1016/j.jiec.2013.08.023
Norfazilah W, Ismail W, Irfan M, Irwan A, Hanisah N, Muhet A, Hidayah N, Bakar A, Yusop HM, Samah NA (2022) Adsorption behavior of heavy metal ions by hybrid inulin-TEOS for water treatment. Civ Eng J 8(09):1787–1798
Okuda T, Baes AU, Nishijima W, Okada M (2001) Coagulation mechanism of salt solution-extracted active component in Moringa oleifera seeds. Water Res 35(3):830–834. https://doi.org/10.1016/S0043-1354(00)00296-7
Peña-Guzmán C, Ortiz-Gutierrez BE (2022) Evaluation of three natural coagulant from Moringa oleifera seed for the treatment of synthetic greywater. Civ Eng J 8(12):3842–3853
Ramavandi B (2014) Treatment of water turbidity and bacteria by using a coagulant extracted from plantago ovata. Water Resour Ind 6(August):36–50. https://doi.org/10.1016/j.wri.2014.07.001
Rondeau V, Commenges D, Jacqmin-Gadda H, Dartigues JF (2000) Relation between aluminum concentrations in drinking water and alzheimer’s disease: an 8-year follow-up study. Am J Epidemiol 152(1):59–66. https://doi.org/10.1093/aje/152.1.59
RS2 (2012) Potable water specification, 2nd edn. Rwanda Bureau of Standard, Kigali
Saleem M, Bachmann RT (2019) Journal of industrial and engineering chemistry a contemporary review on plant-based coagulants for applications in water treatment. J Ind Eng Chem 72:281–297. https://doi.org/10.1016/j.jiec.2018.12.029
Šćiban M, Klašnja M, Antov M, Škrbić B (2009) Removal of water turbidity by natural coagulants obtained from chestnut and acorn. Biores Technol 100(24):6639–6643. https://doi.org/10.1016/j.biortech.2009.06.047
Shak KPY, Wu TY (2014) Coagulation–flocculation treatment of high-strength agro-industrial wastewater using natural cassia obtusifolia seed gum: treatment efficiencies and flocs characterization. Chem Eng J 256:293–305. https://doi.org/10.1016/j.cej.2014.06.093
Shak KPY, Wu TY (2015) Optimized use of alum together with unmodified Cassia obtusifolia seed gum as a coagulant aid in treatment of palm oil mill effluent under natural pH of wastewater. Ind Crops Prod 76:1169–1178. https://doi.org/10.1016/j.indcrop.2015.07.072
Shayegan Z, Razzaghi M, Niaei A, Salari D, Tabar MTS, Akbari AN (2013) Sulfur removal of gas oil using ultrasound-assisted catalytic oxidative process and study of its optimum conditions. Korean J Chem Eng 30(9):1751–1759. https://doi.org/10.1007/s11814-013-0097-5
Subramonian W, Ta Yeong Wu, Chai SP (2014) A comprehensive study on coagulant performance and floc characterization of natural Cassia obtusifolia seed gum in treatment of raw pulp and paper mill effluent. Ind Crops Prod 61:317–324. https://doi.org/10.1016/j.indcrop.2014.06.055
Takaoka A, Baptista A, Oliveira M, Guttierres R, Bergamasco R, Fernandes M, Marquetotti A, Vieira S (2017) Protein fractionation of seeds of Moringa oleifera lam and its application in superficial water treatment. Sep Purif Technol 180:114–124. https://doi.org/10.1016/j.seppur.2017.02.040
Teh CY, Ta Yeong Wu, Juan JC (2014) Optimization of agro-industrial wastewater treatment using unmodified rice starch as a natural coagulant. Ind Crops Prod 56:17–26. https://doi.org/10.1016/j.indcrop.2014.02.018
Jing W, Chakraborty T, Xu CC, Ray MB (2019) Treatment train for tailings pond water using Opuntia ficus-indica as coagulant. Sep Purif Technol 211:448–455. https://doi.org/10.1016/j.seppur.2018.09.083
Yan S, Nagendra K, Yeong Ta, Eshwaraiah M, Nagasundara R (2016) Performance of conventional starches as natural coagulants for turbidity removal. Ecol Eng 94:352–364. https://doi.org/10.1016/j.ecoleng.2016.05.082
Younes H, El-Etriby HK, Mahanna H (2021) High removal efficiency of reactive yellow 160 dye from textile wastewater using natural and modified glauconite. Int J Environ Sci Technol. https://doi.org/10.1007/s13762-021-03528-3
Younes H, Mahanna H, El-Etriby HK (2019) Fast adsorption of phosphate (PO4-) from wastewater using glauconite. Water Sci Technol 80(9):1643–1653. https://doi.org/10.2166/wst.2019.410
Zedan T, Mossad M, Fouad M, Mahanna H (2022) Potential application of natural coagulant extraction from walnut seeds for water turbidity removal. Water Pract Technol 17(3):684–698. https://doi.org/10.2166/wpt.2022.019
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by TZ, HM, and MM. The first draft of the manuscript was written by HM. MF revised and commented on the first draft of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Additional information
Editorial responsibility: Chongqing Wang.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mahanna, H., Fouad, M., Zedan, T. et al. Effective turbid water treatment using natural eco-friendly coagulants derived from oat and onion seeds. Int. J. Environ. Sci. Technol. 21, 4773–4787 (2024). https://doi.org/10.1007/s13762-023-05326-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-023-05326-5